The diagnosis of chronic constipation may seem like a simple concept; however, bowel patients can be a challenging group within our pelvic health population. The interesting part about treating these patients is that constipation can result in a variety of complaints. Diaz et al confirmed this in their 2023 research. Their article shared that “Constipation encompasses several subtypes, each with its unique characteristics and underlying factors.”(1) Common complaints can include frequency, size, length, and consistency which can become overwhelming for the practitioner to decide which clinical complaints seem the most important to focus on.
Knowing how to effectively treat these patients and ask the right questions is valuable in the scheme of pelvic floor rehab, secondary to overlapping symptoms and etiology of chronic constipation. Consideration needs to be taken into account for any outside factors that can also contribute to patient complaints. For example, taking different prescription medications, supplements, and eating different foods can all influence the patient's stool frequency and formation (2). Realizing where this issue stems from is the deep-dive question to get a handle on their complaints.
Bowel frequency can be one of the biggest clinical challenges for clinicians to educate patients to master – consistency is key! Understanding what their normal frequency should be, or what it should be now, is part of the clinical judgment skill when setting goals. If your patient is only getting a natural bowel movement urge every 10 days, their goal of daily BMs, at first, should be emotionally readjusted so that they don’t feel like they have failed their rehabilitation goals.
Learning colon physiology helps to understand normal vs abnormal motility and can be helpful in treating your patient’s constipation. In my course, Bowel Pathology and Physiology we discuss the physiological reflex that controls the lower motility of the GI tract following a meal (3). When patients present with chronic constipation, they may be experiencing a profound loss of the gastrocolic reflex. The course is filled with clinical pearls and research tidbits that help to improve or slow down colonic motility. Join me in the next course offering on September 14-15, 2024 to learn more.
Resources:
- Diaz S, Bittar K, Hashmi MF, et al. [Updated 2023 Nov 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK513291/
- Araújo MM, Botelho PB. Probiotics, prebiotics, and synbiotics in chronic constipation: Outstanding aspects to be considered for the current evidence. Front Nutr. 2022 Dec 8;9:935830. doi: 10.3389/fnut.2022.935830. PMID: 36570175; PMCID: PMC9773270.
- Mawer S, Alhawaj AF. Physiology, Defecation. 2023 Nov 13. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan–. PMID: 30969554.
AUTHOR BIO
Lila Abbate, PT, DPT, MS, OCS, WCS, PRPC
 Lila Abbate is the Director/Owner of New Dimensions Physical Therapy. She graduated from Touro College in Dix Hills, NY with a Bachelor of Science (BS) in Health Sciences and a Master of Arts (MA) in Physical Therapy in 1997. She completed her Advanced Masters in Manual Orthopedic Physical Therapy (MS) at Touro College, Bayshore, NY in 2003 and continued to pursue her Doctor of Physical Therapy (DPT) at Touro in 2005. Dr. Abbate is a Board-Certified Specialist by the American Physical Therapy Association in Orthopedics (OCS) 2004 and Women’s Health (WCS) 2011. She obtained the Certified Pelvic Rehabilitation Practitioner (PRPC) from the Herman & Wallace Institute in 2014. She is a Diane Lee/LJ Lee, Integrated Systems Model (ISM) graduate and completed the New York series in 2012.
Lila Abbate is the Director/Owner of New Dimensions Physical Therapy. She graduated from Touro College in Dix Hills, NY with a Bachelor of Science (BS) in Health Sciences and a Master of Arts (MA) in Physical Therapy in 1997. She completed her Advanced Masters in Manual Orthopedic Physical Therapy (MS) at Touro College, Bayshore, NY in 2003 and continued to pursue her Doctor of Physical Therapy (DPT) at Touro in 2005. Dr. Abbate is a Board-Certified Specialist by the American Physical Therapy Association in Orthopedics (OCS) 2004 and Women’s Health (WCS) 2011. She obtained the Certified Pelvic Rehabilitation Practitioner (PRPC) from the Herman & Wallace Institute in 2014. She is a Diane Lee/LJ Lee, Integrated Systems Model (ISM) graduate and completed the New York series in 2012.
Dr. Abbate has been an educator for most of her physical therapy career. She has experience as a full-time faculty at Touro College, Manhattan Campus from 2002 to 2006 teaching the biomechanical approach to orthopedic dysfunction and therapeutic exercise as well as massage/soft tissue work that highlighted trigger point work, scar management, and myofascial release. She is currently on faculty as a Lecturer at Columbia University teaching the private practice section Business & Management course (since 2016) along with the Pelvic Health elective (since 2012). She teaches nationally and internationally with the Herman & Wallace Pelvic Rehabilitation Institute teaching advanced courses of her own intellectual property: Orthopedic Assessment for the Pelvic Health Therapist, Bowel Pathology Function, Dysfunction and the Pelvic Floor, Coccydynia & Painful Sitting: Orthopedic Implications. She was a co-writer for the Pudendal Neuralgia course and teaches the Pelvic Function Series, and Pregnancy/Postpartum Series. She has written two book chapters in 2016: Pelvic Pain Management by Valvoska and Healing in Urology: Clinical Guidebook to Herbal and Alternative Therapies by Chughtai.
Lila is a member of the American Physical Therapy Association, the National Vulvodynia Association, the American Urogynecology Association, the International Pelvic Pain Society, and is also a Senior Physical Therapy consultant for SI Bone, a sacroiliac joint instrumentation company.

Blog by Deanna Vaughn, PT, DPT who practices at Core and Pelvic Physical Therapy Clinic in Conway, Arkansas, this article was originally located at https://whatsupdownthere.info/colorectal-cancer-the-gut-and-the-butt/.
Colorectal cancer refers to cancerous cells within the colon or rectum. Need a quick anatomy review? Keep reading then!
The colon is another name for the large intestine, which is the long tube (nearly 5 FEET!) surrounding the small intestines (that snaky, jumbled tube in the middle of our bodies, which you can see below in the picture). It’s comprised of segments: the cecum (the little pouch that joins the small intestine to the large intestine) in the right lower abdomen, the ascending colon starting at the right lower part of your abdomen (coming off the cecum), and up to about the right side of your ribcage; the transverse colon that loops underneath the stomach and ribcage from right to left; the descending colon that extends down from the left side of your ribcage to the lower part of your left abdomen; and then the sigmoid colon that loops (in an s-shape) along the lower abdomen to the center of the body. At the end of the colon is the rectum, which pretty much connects the colon to the actual anus/anal opening for wastes to leave the body.
That being said, colorectal cancer can affect any part or segment of the colon and the rectum. If you have a family history of colorectal cancer, or if you have an inflammatory bowel disease (like Crohn’s disease or ulcerative colitis), then you may be at a higher risk for colorectal cancer. Other risk factors are the same for virtually any other health condition – genetics, no regular physical activity, poor diet, tobacco use, high alcohol consumption, etc.
So how would we know if it’s colorectal cancer – or precancerous cells, and how do we decrease our risk?
That’s where screening comes into play! Just like how someone may see their gynecologist annually and undergo the PAP smear every 1-3 years to check for any gynecological cancer (like cervical or labial cancer), someone may see their colorectal or gastrointestinal (GI) provider to check for colorectal cancer or disorders. Regular screening takes place around age 45 (although a person may be screened earlier if they are at higher risk or had a previous history of cancer).
What does screening look like?
There are a few tests that screen for colorectal cancer. These tests include stool tests, flexible sigmoidoscopy, and colonoscopy.
Stool tests – This pretty much involves you taking a sample of your stool via test kit provided to you, and returning it to your doctor/lab, where your stool is checked for any blood or other abnormal findings.
Flexible sigmoidoscopy – A thin, short tube with a light is inserted into the rectum. This allows your doctor to see any polyps or cancer within the rectum and lower part of the colon.
Colonoscopy – This is like the sigmoidoscopy, but with a longer tube. The longer tube allows your doctor to check for polyps/cancer inside the rectum and the entire length of the colon. Your doctor can also remove some polyps during this procedure if indicated.
Most people without any symptoms, abnormal findings or outstanding personal or family history of colorectal cancer will have these screening tests performed anywhere from 5-10 years.
What are the symptoms?
This is not an exhaustive list, but some symptoms may include:
- Bleeding, pain, and/or discomfort within the rectum/anus
- Blood in stool
- Abdominal pain and bloating
- Nausea/vomiting
- Difficulty or incomplete bowel evacuation
- Hemorrhoids
- Altered bowel habits (such as sudden constipation, diarrhea, change in stool consistency)
Now what are our treatment options?
Besides preventative measures – such as getting regular physical activity, improving our diet, etc., treatment looks similar to any other cancer treatment. This may look like chemotherapy, radiation therapy, immunotherapy, and/or surgery. Surgery may be indicated to remove polyps/tumors, or parts of the colon or rectum to eliminate cancerous growths. Thankfully though, regular screening of the colorectal region can find precancerous/cancerous cells early. Oftentimes, such as during a colonoscopy, your colorectal provider may go ahead and remove polyps that are abnormal or deemed precancerous at that time!
Now what about pelvic physical therapy? Can it possibly help?
Well, this is another condition (like Pelvic Congestion Syndrome in the previous blog post), where pelvic physical therapy is not the initial go-to or main treatment option. Individuals with colorectal cancer vary in several ways depending on staging/severity and overall health. Once again, pelvic therapy is a nice resource to utilize if you’re needing or wanting ways to manage your bowel symptoms.
Ways that pelvic PT CAN help may include: Teaching appropriate toileting – positioning to straighten out the anorectal angle and allow stool to pass more easily from the rectum; mechanics, such as exhaling smoothly when pushing for a bowel movement to prevent straining; Improving pelvic floor muscle function (strength, endurance, coordination) so that your body can delay defecation as needed and calm down bowel urges; and overall promoting health bowel habits by supporting your nutrition and keeping bowel movements regular.
Whether or not you (or someone you know) have colorectal cancer, developing healthy and safe bowel habits is key to a better quality of life. Working with your doctor and/or your team of providers is important in making sure your needs are addressed, but feel free to reach out to your local pelvic PT if you want more resources or guidance – even things like, “So, how SHOULD I be pooping??”
References & Resources
Brenner H, Chen C. The colorectal cancer epidemic: challenges and opportunities for primary, secondary and tertiary prevention. Br J Cancer. 2018;119(7):785-792. doi:10.1038/s41416-018-0264-x
https://www.cancer.org/cancer/colon-rectal-cancer.html
https://my.clevelandclinic.org/health/diseases/14501-colorectal-colon-cancer
Kuipers EJ, Grady WM, Lieberman D, et al. Colorectal cancer. Nat Rev Dis Primers. 2015;1:15065. Published 2015 Nov 5. doi:10.1038/nrdp.2015.65
Leslie A, Steele RJC. Management of colorectal cancerPostgraduate Medical Journal 2002;78:473-478. http://dx.doi.org/10.1136/pmj.78.922.473
Mármol I, Sánchez-de-Diego C, Pradilla Dieste A, Cerrada E, Rodriguez Yoldi MJ. Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer. Int J Mol Sci. 2017;18(1):197. Published 2017 Jan 19. doi:10.3390/ijms18010197
You YN, Lee LD, Deschner BW, Shibata D. Colorectal Cancer in the Adolescent and Young Adult Population. JCO Oncol Pract. 2020;16(1):19-27. doi:10.1200/JOP.19.00153
Lila Abbate PT, DPT, OCS, WCS, PRPC is the creator and instructor of Bowel Pathology Function & Dysfunction and the Pelvic Floor, a course which instructs in comprehensive evaluation and treatment techniques for bowel pathologies and dysfunctions, including fecal incontinence, chronic constipation, and the relationship between constipation and rectal and/or abdominal pain. Join Dr. Abbate in one of five events taking place in 2020!
Bowel dysfunction can be very rewarding to treat. Most pelvic health physical therapists are nervous about diving into bowel treatment. When I was training with my mentor, Elise Stettner, PT she used to remind me that “any PT can treat urinary symptoms. The patients who are really suffering are those bowel dysfunctions.” That statement really stuck with me and mentoring with her and treating those patients created a passion for treating patients who suffer from bowel dysfunction.
 Within the term bowel dysfunction, fecal urgency, is a common symptom and is under-researched. In 2019, Similis, et al published A Systemic Review and Network Meta-Analysis Comparing Treatments for Faecal Incontinence, doesn’t even mention physical therapy and pelvic floor muscle rehabilitation as an intervention for fecal incontinence and fecal urgency treatment.
Within the term bowel dysfunction, fecal urgency, is a common symptom and is under-researched. In 2019, Similis, et al published A Systemic Review and Network Meta-Analysis Comparing Treatments for Faecal Incontinence, doesn’t even mention physical therapy and pelvic floor muscle rehabilitation as an intervention for fecal incontinence and fecal urgency treatment.
Anecdotally, I have a lot of pelvic health patients and even generalized orthopedic patients who report that having bowel urgency is a more apparent symptom in their life after having a back or hip surgery. What started as a once-in-a-while problem, fecal urgency has crept up and become the new normal in their lives. They have subliminally re-routed their day to accommodate their bowel movements in order avoid incidences and accidents whether its waiting to eat breakfast until they get to work, waiting to drink a favorite drink until they are near a toilet or taking supplements before bed to empty their bowels before they start their day in order to avoid accidents during their day. Learning to treat bowel urgency can tremendously help patients regain control and abolish their symptoms.
Bowel urgency has many parallels to urinary urgency. The colon is giving the signal too soon, potentially at an inappropriate time, and the muscles need to be strong enough to hold the urge of defecation back in order to postpone. The failure occurs when one part of the continence mechanism fails. Bowel Pathology Function & Dysfunction and the Pelvic Floor course helps you to learn how to treat and guide your patients and conquer all types of bowel dysfunction.
Similis et al, A systematic review and network meta-analysis comparing treatments for faecal incontinence. Int J Surg. 2019 Jun;66:37-47. doi: 10.1016/j.ijsu.2019.04.007. Epub 2019 Apr 22.
For over 25 years my practice has had a focus on children suffering from bloating, gas, abdominal pain, fecal incontinence and constipation. Functional Gastrointestinal Disorders (FGID) are disorders of the brain -gut interaction causing motility disturbance, visceral hypersensitivity, altered immune function, gut microbiota and CNS processing. (Hyams et al 2016). Did you know that children who experience chronic constipation that do not get treated have a 50% chance of having issues for life?
 The entire GI system is as amazing as it is and complicated. Its connection to the nervous system is fascinating, making it a very sensitive system. In her book GUT, Giulia Enders talks about Ninety percent of the serotonin we need comes from our gut! The psychological ramifications of ignoring the problem are too great (Chase et el 2018). Last year an 18-year-old patient of mine had to decline a scholarship to an Ivy League University because she needed to live at home due to her bowel management problem.
The entire GI system is as amazing as it is and complicated. Its connection to the nervous system is fascinating, making it a very sensitive system. In her book GUT, Giulia Enders talks about Ninety percent of the serotonin we need comes from our gut! The psychological ramifications of ignoring the problem are too great (Chase et el 2018). Last year an 18-year-old patient of mine had to decline a scholarship to an Ivy League University because she needed to live at home due to her bowel management problem.
Unfortunately, FGID conditions can lead to suicide and death. Over 15 years ago my children’s pediatrician told me about an 11-year-old boy who hung himself because he had encopresis. In 2016 a 16-year-old girl suffered a cardiac arrest and died because of constipation.
The problems with children are different than for adults and need to be addressed with a unique approach.
How do physical therapists treat pediatric FGID?
- Have a solid foundation in the gastrointestinal system
- Coordinate muscle functions from top to bottom!
- Identify common childhood patterns
- Learn treatment techniques and strategies to address the issues specifically
Study and understand gastrointestinal anatomy, physiology, function and examination techniques. The entire GI system is as amazing as it is complicated. Its connection to the nervous system is fascinating, making it a very sensitive system. Ninety percent of the serotonin we need comes from our gut! The psychological ramifications of ignoring the problem are too great.
What do the Pelvic Floor Muscles (PFM) have to do with it?
Encopresis leads to a weak internal/external anal sphincter and pelvic floor muscles and constipation leads to pelvic floor muscles that can’t relax. Confused? When the Rectal Anal Inhibitory Reflex or RAIR fails from bypass diarrhea the sphincter muscles relax, and feces leaks out. This constant leakage leads to weak sphincter and pelvic floor muscles. When it happens on a regular basis most children don’t feel it, however their peers smell it and life changes.
My course, Pediatric Functional Gastrointestinal Disorders, teaches how to coordinate the muscle function based on the tasks required.
How did this start?
One painful bowel movement can lead to withholding for the next due to fear of the pain happening again. The muscles of the pelvic floor then tighten to hold the poop in. This actually does not make the muscle strong but instead makes it confused. The muscle then is controlled by the consistency of poop being too hard and painful to let out or too loose and not able to hold in.
Managing functional GI disorders is a process. It takes the bowel a long time to re-train and it requires patience and skill to know how to do it. Many therapists and patients themselves get frustrated and compliance fails. This is mostly due to lack of knowing how to titrate medications and give the bowel what it needs (other than proper nutrition that is!) It's like retraining a person to walk after a stroke, the brain needs to relearn normal bowel sensations.
Most families don’t realize how severe constipation can be. It is an insidious problem that gets ignored until it is too late.
Typically, what I hear from parents is their child was diagnosed with constipation and was advised to take a daily laxative. So, which one is the best one? How do they all work? Once leakage occurs again the laxative is discontinued as we think the bowel must be empty and this medication is causing the leaks which is counterproductive. Now the frustrating cycle of backing up or being constipated begins again. The constipation returns, the laxative is restarted, the loose stool leaks out and the laxative is stopped and that is the REVOLVING DOOR or what I refer to as children riding the “Constipation Carousel”. The bowel is an amazingly beautiful, smart but also sensitive organ that does not like this back and forth and therefore will not learn how to be normal. In the meantime, they experience distended abdomens and dysmorphia ending up in eating disorder clinics. I had an 11-year-old girl taking Amitriptyline for abdominal pain all because of a pressure problem in the gut not knowing how to work the pelvic floor with the diaphragm and her core.
No two children are the same and no two colons are the same. Laxatives need to be titrated to the specific needs of your child’s colon and motility of their colon not their age or body weight.
The success in getting children to have regular bowel movements of normal consistency without any fecal leaks is based not only teaching how to titrate laxatives but also how to sense urge, become aware of the pelvic floor muscles and learn how NOT to strain to defecate, retrain the core and diaphragm with the ribcage and integrate developmental strategies for function. Teaching Interoception- what my body feels like when I have an urge- is an important part of this course. This is especially important for those children born with anorectal malformations or congenital problems such as imperforate anus or Hirschsprung’s Disease.
In this class we use visceral techniques, manual therapy techniques, sensory techniques and neuromuscular reeducation and coordination to retrain the entire system.
Come and explore the amazing gut with me and learn how to improve the health and well-being of your patients, in Pediatric Functional Gastrointestinal Disorders!
1. Hyams, JS, et al. Childhood Functional Gastrointestinal Disorders: Child/Adolescent. Gastroenterology volume 150, 2016;1456-1468.
2. Drossman DA. Functional gastrointestinal disorders: history, pathophysiology, clinical features and rome IV. Gastroenterology 2016;150:1262-1279
3. Robin SG, Keller C, Zwiener R, et al. Prevalence of Pediatric Functional Gastrointestinal Disorders Utilizing the Rome IV Criteria. J Pediatr 2018; 195:134.
4. Koppen IJN, Vriesman MH, Saps M, Rajindrajith S, Shi X, van Etten-Jamaludin FS, Di Lorenzo C, Benninga MA, Tabbers MM. Prevalence of Functional Defecation Disorders in Children: A Systematic Review and Meta-Analysis. J Pediatr. 2018 Jul;198:121-130.e6. doi: 10.1016/j.jpeds.2018.02.029. Epub 2018 Apr 12.
5. Zar-Kessler C, Kuo B, Cole E, Benedix A, Belkind-Gerson, J. Benefit of pelvic floor physical therapy in pediatric patients with dyssynergic defecation constipation. 2019 Dig Dis https://doi.org/10.1159/000500121/
6. Chase J, Bower W, Susan Gibb S. et al. Diagnostic scores, questionnaires, quality of life, and outcome measures in pediatric continence: A review of available tools from the International Children’s Continence Society. J Ped Urol (2018) 14, 98e107
I recently found this article from the Psychology Research and Behavior Management Journal. I found myself curious about how other healthcare disciplines treat a diagnosis that often presents in conjunction with pelvic floor dysfunction. Irritable bowel syndrome, or IBS, affects nearly 35 million Americans. It is considered a ‘functional’ condition meaning that symptoms occur without structural or biochemical pathology. There is often a stigma with functional diagnosis that the symptoms are “all in their heads”, and while there are many theories about what predisposes individuals to IBS, the experts now think of IBS as a “disorder of gut brain interaction”. Generally, there are 3 subtypes of IBS where people note either constipation dominant, diarrhea dominant or mixed. In order to be diagnosed an individual must report abdominal pain at least 1 day per week in the last 3 months which is related to stooling and a change in frequency or form. Other symptoms that are common are bloating, nausea, incomplete emptying, and urgency.
The author suggests a biopsychosocial framework to help understand IBS. An interdependent relationship between biology (gut microbiota, inflammation, genes), behavior (symptom avoidance, behaviors), cognitive processes (“brain-gut dysregulation, visceral anxiety, coping skills”), and environment (trauma, stress). The brain-gut connection by a variety of nerve pathways is how the brain and gut communicate in either direction; top down or bottom up. Stress and trauma can dysregulate gut function and can contribute to IBS symptoms.
Stress affects the autonomic nervous system that contributes to sympathetic (fight/flight) and parasympathetic (rest/digest). Patients with IBS may have dysfunction with autonomic nervous system regulation. Symptoms of dysregulated gut function can present as visceral hypersensitivity, visceral sensitivity, and visceral anxiety. Visceral hypersensitivity is explained as an upregulation of nerve pathways. The author sites studies that note that IBS patients have a lower pain tolerance to rectal balloon distention than healthy controls. Visceral sensitivity is another sign of upregulation where IBS patients have a greater emotional arousal to visceral stimulation and are less able to downregulate pain. The author notes that the IBS population show particular patterns of anxiety with visceral anxiety and catastrophizing. Visceral anxiety is described hypervigilance to bowel movements and fear avoidance of situational symptoms. For example, fear of not knowing where the bathroom is located.
Cognitive behavioral therapy (CBT) has been shown to be an effective treatment to decrease the impact of IBS symptoms. CBT is focused on modifying behaviors and challenging dysfunctional beliefs. CBT can be presented in a variety of ways, however most include techniques consisting of education of how behaviors and physiology interplay for example the gut and stress response; relaxation strategies, usually diaphragmatic breathing and progressive relaxation; cognitive restructuring to help individuals see the relationship between thought patterns and stress responses; problem-solving skills with shift to emotion focused strategies (“acceptance, diaphragmatic breathing, cognitive restructuring, exercise, social support”) instead of problem focused strategies, and finally exposure techniques to help the individual slowly face fear avoidance behaviors. So much of the techniques are similar to what pelvic floor therapists try to educate our patients in. It is reassuring to me that through we may be different disciplines we are on the same team are moving towards the same goal. The author recommends a 10 session treatment duration, and notes that may be a barrier for some. Integrated practice with other healthcare professionals is also recommended. The more we can know about what our other team members are doing to help support patients the more effective we all are.
Kinsinger, Sarah W. “Cognitive-behavioral therapy for patients with irritable bowel syndrome: current insights” Psychology research and behavior management vol. 10 231-237. 19 Jul. 2017, doi:10.2147/PRBM. S120817
“What's wrong with children?”
As pelvic health physical therapists we take care of people suffering from bladder and bowel incontinence and/or dysfunction as well as pre-natal/ post-partum back pain, weak core muscles and pelvic pain. I was approached over 30 years ago by a urologist to take care of his pediatric patients. My reply: “What’s wrong with children?” It’s been a whirlwind of learning since that day!
Pediatric pelvic floor dysfunction is common and can have significant consequences on quality of life for the child and the family, as well as negative health consequences to the lower urinary tract if left untreated.
 According to the National Institute of Diabetes and Digestive and Kidney Diseases, by 5 years of age, over 90% of children have daytime bladder control (NIDDK, 2013) What is life like for the other 10% who experience urinary leakage during the day?
According to the National Institute of Diabetes and Digestive and Kidney Diseases, by 5 years of age, over 90% of children have daytime bladder control (NIDDK, 2013) What is life like for the other 10% who experience urinary leakage during the day?
Bed-wetting is also a pediatric issue with significant negative quality of life impact for both children and their caregivers, with as much as 30% of 4-year-olds experiencing urinary leakage at night (Neveus, 2010). Children who experience anxiety-causing events may have a higher risk of developing urinary incontinence, and in turn, having incontinence causes considerable stress and anxiety for children (Austin, 2014; Neveus, 2010).
Additionally, bowel dysfunction, such as constipation, is a contributor to urinary leakage or urgency. With nearly 5% of pediatric office visits occurring for constipation (Thibodeau 2013, NIDDK, 2013), the need to address these issues is great! And, since pediatric bladder and bowel dysfunction can persist into adulthood, we must direct attention to the pediatric population to improve the health of all our patients.
Children suffer from many diagnoses that affect the pelvic floor including (Austin et al, 2014);
- Voiding dysfunction
- Enuresis (Bedwetting)
- Daytime urinary incontinence
- Urinary urgency and frequency
- Vesicoureteral reflux (Backflow of urine into the kidney)
- Pelvic pain (yes pelvic pain!)
The most common diagnoses I treat are voiding dysfunction and constipation. Pediatric voiding dysfunction is defined as involuntary and intermittent contraction or failure to relax the urethral muscles while emptying the bladder. (Austin et al, 2014); The dysfunctional voiding can present with variable symptoms including urinary urgency, urinary frequency, incontinence, urinary tract infections, and vesicoureteral reflux. Frequently, constipation is a culprit or cause. (Austin et al, 2014; Hodges S. 2012); Managing constipation can have a very positive effect on voiding dysfunction.
“What do we do to teach the pelvic floor (Kegel) muscles to work?”
Common questions I am asked include:
- Can I use biofeedback with children?
- Do we complete internal assessments on pediatric patients?
- How do we teach kids so they can understand?
- Do kids have the ability to learn strengthening versus relaxation?
- How do you teach a child to become aware of their pelvic floor and coordinate it?
If you have pondered these questions, let’s delve in! I see children as young as 4 who have been able to master biofeedback and recite back to me how their pelvic floor works with bowel and bladder function! Children are so eager to please and they love working with animated biofeedback sessions. The research supports the potential benefit of biofeedback training for children with pelvic floor dysfunction (DePaepe et al. 2002, Kaye 2008, Kajbafzadeh 2011, Fazeli 2014). The children are engaged and learn how to isolate their pelvic floor muscles (PFM) through positioning and breathing. The exercises are fun and easy to do. We also incorporate the core! What a wonderful opportunity we have to educate the younger population on these vital muscles as well as proper diet and bowel/bladder habits!
It is not typical to complete an internal pelvic muscle assessment on children, as this would not be appropriate.
“How do I treat it?”
In the literature on pediatric bowel and bladder dysfunction you will often come across the word "Urotherapy". It is, by definition, a conservative management-based program used to treat lower urinary tract (LUT) dysfunction. (Austin 2014)
Basic Urotherapy includes education on the anatomy, behavior modifications including fluid intake, timed or scheduled voids, toileting postures and avoidance of holding maneuvers, diet, avoiding bladder irritants and constipation. Parents are often not aware of their children’s voiding habits once they are cleared from diaper duty after successful potty training occurs.
Urotherapy alone can be helpful however a recent study (Chase, 2010) demonstrated a much greater improvement in those patients who received pelvic floor muscle training as compared to Urotherapy alone.
The International Children’s Continence Society (ICCS) has now expanded the definition of Urotherapy to include Specific Urotherapy (Austin et al, 2014). This includes biofeedback of the pelvic floor muscles by a trained professional who can teach the child how to alter pelvic floor muscle activity specifically for voiding. Cognitive behavioral therapy and psychotherapy are also important and can be a needed in combination with biofeedback in specific cases.
As you can see, PFM exercise combined with Urotherapy is a safe, inexpensive, and effective treatment option for children with pediatric voiding dysfunction.
Do bladder and bowel problems cause psychological problems or is the reverse true?
When we think of pediatric bowel and bladder issues, we primarily focus on what is happening to cause the bowel or bladder leakage and treat it accordingly. It is imperative to teach a child that she/he did not have an “accident”, but their bladder or bowel had a leak. It makes the incident a physiological problem and not something they did. See my blog post on “Accident” for more information.
It is not always apparent how much the child is suffering from issues with self-esteem, embarrassment, internalizing behaviors, externalizing behaviors or oppositional defiant disorders. Dr. Hinman recognized theses issues years ago (1986) and commented that voiding dysfunctions might cause psychological disturbances rather than the reverse being true. Dr. Rushton in 1995 wrote that although a high number of children with enuresis are maladjusted and exhibit measurable behavioral symptoms, only a small percentage have significant underlying psychopathology. In other more recent studies (Joinson et al. 2006a, 2006b, 2008, Kodman-Jones et al, 2001) it was noted that elevated psychological test scores returned to normal after the urologic problem was cured.
I frequently get testimonials from my patients. I would say the common denominator is the child and/or caregivers report that the child is “much better adjusted,” “happier”, “come out of his shell”, “more outgoing”, “making friends.” As a side note -- they’re happy they don’t leak anymore.
You can learn more about treating pediatric patients in my courses,
Pediatric Incontinence and Pelvic Floor Dysfunction and Pediatric Functional Gastrointestinal Disorders.
Austin, P., Bauer, S.B., Bower, W., et al. The standardization of terminology of lower urinary tract function in children and adolescence: update report from the standardization committee of the international children’s continence society. J Urol (2014) 191.
Chase J, Austin P, Hoebeke P, McKenna P. The management of dysfunctional voiding in children: a report from the standarisation committee of the international children’s continence society. 2010; J Urol183:1296-1302.
Constipation in Children. (2013)retrieved June 9, 2014 from http://kidney.niddk.nih.gov/kudiseases/pubs/uichildren/index.aspx
DePaepe H., Renson C., Hoebeke P., et al: The role of pelvic- floor therapy in the treatment of lower urinary tract dysfunctions in children. Scan J of Urol and Neph 2002; 36: 260-7.
Farahmand, F., Abedi, A., Esmaeili-dooki, M. R., Jalilian, R., & Tabari, S. M. (2015). Pelvic Floor Muscle Exercise for Paediatric Functional Constipation.Journal of Clinical and Diagnostic Research : JCDR, 9(6), SC16–SC17. http://doi.org/10.7860/JCDR/2015/12726.6036
Fazeli MS, Lin Y, Nikoo N, Jaggumantri S1, Collet JP, Afshar K. Biofeedback for Non-neuropathic daytime voiding disorders in children: A systematic review and meta-analysis of randomized controlled trials. J Urol. 2014 Jul 26. pii: S0022-5347(14)04048-8.
Hinman, F. Nonneurogenic neurogenic bladder (the Hinman Syndrome)-15 years later. J Urol 1986;136, 769-777.
Hodges SJ, Anthony E. Occult megarectum:a commonly unrecognized cause of enuresis. Urology. 2012 Feb;79(2):421-4. doi: 10.1016/j.urology.2011.10.015. Epub 2011 Dec 14.
Hoebeke, P., Walle, J. V., Theunis, M., De Paepe, H., Oosterlinck, W., & Renson, C. Outpatient pelvic-floor therapy in girls with daytime incontinence and dysfunctional voiding. Urology 1996; 48, 923-927.
Joinson, C., Heron, J., von Gontard, A. and the ALSPAC study team: Psychological problems in children with daytime wetting. Pediatrics 2006a; 118, 1985-1993.
Joinson, C., Heron, J., Butler, U., von Gontard, A. and the ALSPAC study team: Psychological differences between children with and without soiling problems. Pediatrics 2006b; 117, 1575-1584.
Joinson, C., Heron, J., von Gontard, A., Butler, R., Golding, J., Emond, A.: Early childhood risk factors associated with daytime wetting and soiling in school-age children. Journal of Pediatric Psychology2008; e-published.
Kajbafzadeh AM, harifi-Rad L, Ghahestani SM, Ahmadi H, Kajbafzadeh M, Mahboubi AH. (2011) Animated biofeedback: an ideal treatment for children with dysfunctional elimination syndrome. J Urol;186, 2379-2385.
Kaye JD, Palmer LS (2008) Animated biofeedback yields more rapid results than nonanimated biofeedback in the treatment of dysfunctional voiding in girls. J Urol 180, 300-305
Kodman-Jones, C., Hawkins, L., Schulman, SL. Behavioral characteristics of children with daytime wetting. J Urol 2001;Dec(6):2392-5.
Neveus, T, Eggert P, Evans J, et al. Evaluation of the treatment for monosymptomatic enuresis: a standarisation document from the international children’s continence society. J Urol 2010; 183: 441-447
Rushton, H. G. Wetting and functional voiding disorders. Urologic Clinics of North America, 1995; 22(1), 75-93.
Seyedian, S. S. L., Sharifi-Rad, L., Ebadi, M., & Kajbafzadeh, A. M. (2014). Combined functional pelvic floor muscle exercises with Swiss ball and urotherapy for management of dysfunctional voiding in children: a randomized clinical trial. European Journal of Pediatrics, 173(10), 1347-1353.
Thibodeau, B. A., Metcalfe, P., Koop, P., & Moore, K. (2013). Urinary incontinence and quality of life in children. Journal of pediatric urology, 9(1), 78-83.
Urinary Incontinence in Children. (2012). Retrieved June 9, 2014 from http://kidney.niddk.nih.gov/kudiseases/pubs/uichildren/index.aspx
Zivkovic V, Lazovic M, Vlajkovic M, Slavkovic A, Dimitrijevic L, Stankovic I, Vacic N. (2012). Diaphragmatic breathing exercises and pelvic floor retraining in children with dysfunctional voiding. European Journal of Physical Rehabilitation Medicine. 48(3):413-21. Epub 2012 Jun 5.
Andrea Wood, PT, DPT, WCS, PRPC is a pelvic health specialist at the University of Miami downtown location. She is a board certified women’s health clinical specialist (WCS) and a certified pelvic rehabilitation practitioner (PRPC). She is passionate about orthopedics and pelvic health. In her spare time, you can find her enjoying the south Florida outdoors.
Inflammatory bowel disease (IBD) includes the two diagnosis of Crohn’s Disease and Ulcerative Colitis. While both can cause similar health effects, the differences of the disease pathologies are listed below:1
| Ulcerative Colitis | Crohn’s Disease | |
| Affected Area |
|
|
| Pattern of Damage |
|
|
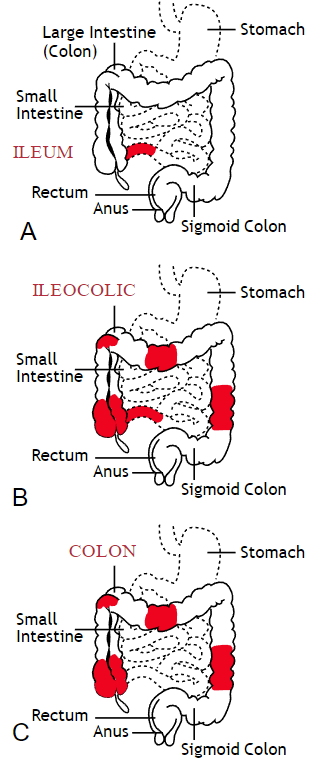
Common complications experienced by patients with IBD include fecal incontinence, fecal urgency, night time soiling, urinary incontinence, abdominal pain, hip and core weakness, pelvic pain, fatigue, osteoporosis, and sarcopenia. In a sample of 1,092 patients with Crohn’s Disease, Ulcerative Colitis, or unclassified IBD, 57% reported fecal incontinence. Fecal incontinence was reported not only during periods of flare ups, but also during remission periods.2 One common factor affecting fecal incontinence is external anal sphincter fatigue. External anal sphincter fatigue has also been shown to be present in IBD patients who are not experiencing fecal incontinence or fecal urgency. IBD patients have been shown in studies to have similar baseline pressures versus healthy matched controls, thus indicating the possibility that deficits in endurance versus strength can play a larger role in fecal incontinence.3 Other factors contributing to fecal incontinence include post inflammatory changes that may alter anorectal sensitivity, anorectal compliance, neuromuscular coordination, and cause visceral hypersensitivity. Visceral hypersensitivity may be caused by continuous release of inflammatory mediators found in patients with IBD. It is also important to screen properly for incomplete bowel emptying and stool consistency to rule out overflow diarrhea or fecal impaction. Reports of need to splint digitally for full evacuation may indicate incomplete bowel emptying and defaectory disorders such as paradoxical contraction of the puborectalis muscle or rectocele. Anorectal manometry testing may be highly useful in identifying patients likely to improve from biofeedback therapy.4
Urinary incontinence can also be another secondary consequence to IBD. In a sample of 4,827 patients with IBD, 1/3 of responders reported urinary incontinence that was strongly associated with the presence of fecal incontinence. Frequent toilet visits for defecation may stimulate overactive bladder. Women were more likely to experience fecal incontinence versus men. One possible mechanism for increased fecal incontinence in women is men often have a longer and more complete anal sphincter that may be protective of fecal incontinence.5
Physical activity has been shown to be lower in patients with IBD versus healthy controls. 6, 7 Guiding IBD patients in proper exercises programs can have great benefits. Exercise may reduce inflammation in the gut and maintain the integrity of the intestines, reducing inflammatory bowel disease risk.8 It can also help increase bone mass density, an important factor in IBD patients who are at greater risk for osteoporosis. It has also been shown to help general fatigue in IBD patients. Patients with Crohn’s disease who participate in higher exercise levels may be less likely to develop active disease at 6 months. Treadmill training at 60% VO2 max and running three times a week has not been shown to evoke gastrointestinal symptoms in IBD patients. An increase of BMI predicts poorer outcomes and shorter time to first surgery in patients with Crohn’s disease.6
Conservative physical therapy interventions for treating IBD symptoms can include the following:
| Symptoms resulting from IBD | Physical Therapy Interventions |
| Fecal Incontinence (FI) |
|
| Urinary urgency |
|
| Sarcopenia |
|
| Fatigue |
|
| Pelvic Pain |
|
Surgical interventions for IBD are dependent upon what type of disease the patient has and what areas of the intestines are affected the most. Surgery may be considered once the disease has become non responsive to medication therapy and quality of life continues to decline. A colectomy involves removing the colon while a proctocolectomy involves both removal of the colon and rectum. For ulcerative colitis patients, options include total proctocolectomy with end ileostomy or a restorative proctocolectomy with ileal pouch anal anastomosis. Restorative proctocolectomy eliminates the need for an ostomy bag making it the preferred surgery of choice if possible and gold standard for ulcerative colitis patients.10 For patients with Crohn’s disease, options include resection of part of the intestines followed by an anastomosis of the remaining healthy ends of the intestines, widening of the narrowed intestine in a procedure called a strictureplasty, colectomy or proctocolectomy, fistula repair, and removal of abscesses if needed.11
1. Crohn’s and Colitis Foundation. 2019. What is Crohn’s Disease. Retrieved from: http://www.crohnscolitisfoundation.org/what-are-crohns-and-colitis/what-is-crohns-disease/
2. Vollebregt PF, van Bodegraven A, Markus-de Kwaadsteniet T, et al. Impacts on perianal disease and faecal incontinence on quality of life and employment in 1092 patients with inflammatory bowel disease. Ailment Pharmacol Ther. 2018; 47: 1253-1260
3. Athanasios A, Kostantinos H, Tatsioni A et al. Increased fatigability of external anal sphincter in inflammatory bowel disease: significance in fecal urgency and incontinence. J Crohns Colitis (2010) 4: 553-560.
4. Nigam G, Limdi J, Vasant D. Current perspectives on the diagnosis and management of functional anorectal disorders in patients with inflammatory bowel disease. Therap Adv Gastroenterol. 2018 Dec 6: doi: 10.1177/1756284818816956
5. Norton C, Dibley L, Basset P. Faecal incontinence in inflammatory bowel disease: Associations and effect on quality of life. J Crohn’s Colitis. (2013) 7, e302-e311.
6. Biliski J, Mazur-Bialy A, Brzozowski B et al. Can exercise affect the course of inflammatory bowel disease? Experimental and clinical evidence. Pharmacological Reports. 2016 (68): 827-836.
7. Tew G, Jones K, Mikocka-Walus A. Physical activity habits, limitations, and preditors in people with inflammatory bowel disease: a large cross-sectional online survey. Inflamm Bowel Dis. 2016; 22(12): 2933-2942.
8. Vincenzo M, Villano I, Messina A. Exercise modifies the gut microbiota with positive health effects. Oxidative Medicine and Cellular Longevitiy. 2017: Article ID 3831972.
9. Cramer H, Schafer M, Schols M. Randomised clinical trial: yoga vs written self care advice for ulcerative colitis. Aliment Pharmacol Ther. 2017; 45: 1379-1389.
10. Cornish J, Wooding K, Tan E, et al. Study of sexual, urinary, and fecal function in females following restorative proctocolectomy. Inflamm Bowel Dis. 18 (9) 2012. 1601-160
11. Crohn’s and Colitis Foundation. 2019. Surgery Options. Retrieved from: http://www.crohnscolitisfoundation.org/what-are-crohns-and-colitis/what-is-crohns-disease/surgery-options.html
A question that often comes up in conversation around menopause is that of pelvic health – the effects on bladder, bowel or sexual health…what works, what’s safe, what’s not? Is hormone therapy better, worse or the same in terms of efficacy when compared to pelvic rehab? Do we have a role here?
An awareness of pelvic health issues that arise at menopause was explored in Oskay’s 2005 paper ‘A study on urogenital complaints of postmenopausal women aged 50 and over’ stating ‘…Urinary incontinence and sexual problems, particularly decline in sexual desire, are widespread among postmenopausal women. Frequent urinary tract infections, obesity, chronic constipation and other chronic illnesses seem to be the predictors of UI.’
 Moller’s 2006 paper explored the link between LUTS (Lower Urinary Tract Symptoms) and sexual activity at midlife: the paper discussed how lower urinary tract symptoms (LUTS) have a profound impact on women’s physical, social, and sexual well being, and confirmed that LUTS are likely to affect sexual activity. However, they also found that conversely, sexual activity may affect the occurrence of LUTS – in their study a questionnaire was sent to 4,000 unselected women aged 40–60 years, and they found that compared to women having sexual relationship, a statistically significant 3 to 6 fold higher prevalence of LUTS was observed in women with no sexual relationship. They also found that women who ceased sexual relationship an increase in the de novo occurrence of most LUTS was observed, concluding that ‘…sexual inactivity may lead to LUTS and vice versa’.
Moller’s 2006 paper explored the link between LUTS (Lower Urinary Tract Symptoms) and sexual activity at midlife: the paper discussed how lower urinary tract symptoms (LUTS) have a profound impact on women’s physical, social, and sexual well being, and confirmed that LUTS are likely to affect sexual activity. However, they also found that conversely, sexual activity may affect the occurrence of LUTS – in their study a questionnaire was sent to 4,000 unselected women aged 40–60 years, and they found that compared to women having sexual relationship, a statistically significant 3 to 6 fold higher prevalence of LUTS was observed in women with no sexual relationship. They also found that women who ceased sexual relationship an increase in the de novo occurrence of most LUTS was observed, concluding that ‘…sexual inactivity may lead to LUTS and vice versa’.
So, who advises women going through menopause about issues such as sexual ergonomics, the use of lubricants or moisturisers, or provide a discussion about the benefits of local topical estrogen? As well as providing a skillset that includes orthopaedic assessment to rule out any musculo-skeletal influences that could be a driver for sexual dysfunction? That would be the pelvic rehab specialist clinician! Tosun et al asked the question ‘Do stages of menopause affect the outcomes of pelvic floor muscle training?’ and the answer in this and other papers was yes; with the research comparing pelvic rehab vs hormone therapy vs a combination approach of pelvic rehab and topical estrogen providing the best outcomes. Nygaard’s paper looked at the ‘Impact of menopausal status on the outcome of pelvic floor physiotherapy in women with urinary incontinence’ and concluded that : ‘…(both pre and postmenopausal women) benefit from motor learning strategies and adopt functional training to improve their urinary symptoms in similar ways, irrespective of hormonal status or HRT and BMI category’.
We must also factor in some of the other health concerns that pelvic health can impact at midlife for women – Brown et al asked the question ‘Urinary incontinence: does it increase risk for falls and fractures?’ They answered their question by concluding that ‘‘… urge incontinence was associated independently with an increased risk of falls and non-spine, nontraumatic fractures in older women. Urinary frequency, nocturia, and rushing to the bathroom to avoid urge incontinent episodes most likely increase the risk of falling, which then results in fractures. Early diagnosis and appropriate treatment of urge incontinence may decrease the risk of fracture.’
If you are interested in learning more about pelvic health, sexual function and bone health at Menopause, consider attending Menopause Rehabilitation and Symptom Management.
Sexual activity and lower urinary tract symptoms’ Møller LA1, Lose G. Int Urogynecol J Pelvic Floor Dysfunct. 2006 Jan;17(1):18-21. Epub 2005 Jul 29.
A study on urogenital complaints of postmenopausal women aged 50 and over. Oskay UY1, Beji NK, Yalcin O. Acta Obstet Gynecol Scand. 2005 Jan;84(1):72-8.
Do stages of menopause affect the outcomes of pelvic floor muscle training? Tosun ÖÇ1, Mutlu EK, Tosun G, Ergenoğlu AM, Yeniel AÖ, Malkoç M, Aşkar N, İtil İM. Menopause. 2015 Feb;22(2):175-84. doi: 10.1097/GME.0000000000000278.
‘Impact of menopausal status on the outcome of pelvic floor physiotherapy in women with urinary incontinence.’ Nygaard CC1, Betschart C, Hafez AA, Lewis E, Chasiotis I, Doumouchtsis SK. Int Urogynecol J. 2013 Dec;24(12):2071-6. doi: 10.1007/s00192-013-2179-7. Epub 2013 Jul 17
After completing an intake on a patient and learning that her history of constipation started about 3 years ago with insidious onset, the story wasn’t really making any sense of how or why this started. Yes, she was menopausal. Yes, she seemed to be eating fiber and drinking water. Yes, she got a bowel movement urge daily, but her bowel movements felt incomplete. Yes, she was a little older, using Estrace cream, and her mobility had slowed down, but nothing seemed to make sense in the story that was leading me to believe it was an emptying problem or a stool consistency issue. She had a bowel movement urge, she could empty, but it was incomplete.
 So, after explaining about physical therapy, the muscle problems involved and what we do here, it led us to the physical examination portion. I explained that we check both the vaginal and rectal pelvic floor muscle compartments to determine rectal fullness internally, check for a rectocele, check for muscle lengthening (excursion) and shortening (contraction). She was on board and desperate to find an answer. She was eager for me to help her find an answer to her emptying problem that she had for the last 3 years.
So, after explaining about physical therapy, the muscle problems involved and what we do here, it led us to the physical examination portion. I explained that we check both the vaginal and rectal pelvic floor muscle compartments to determine rectal fullness internally, check for a rectocele, check for muscle lengthening (excursion) and shortening (contraction). She was on board and desperate to find an answer. She was eager for me to help her find an answer to her emptying problem that she had for the last 3 years.
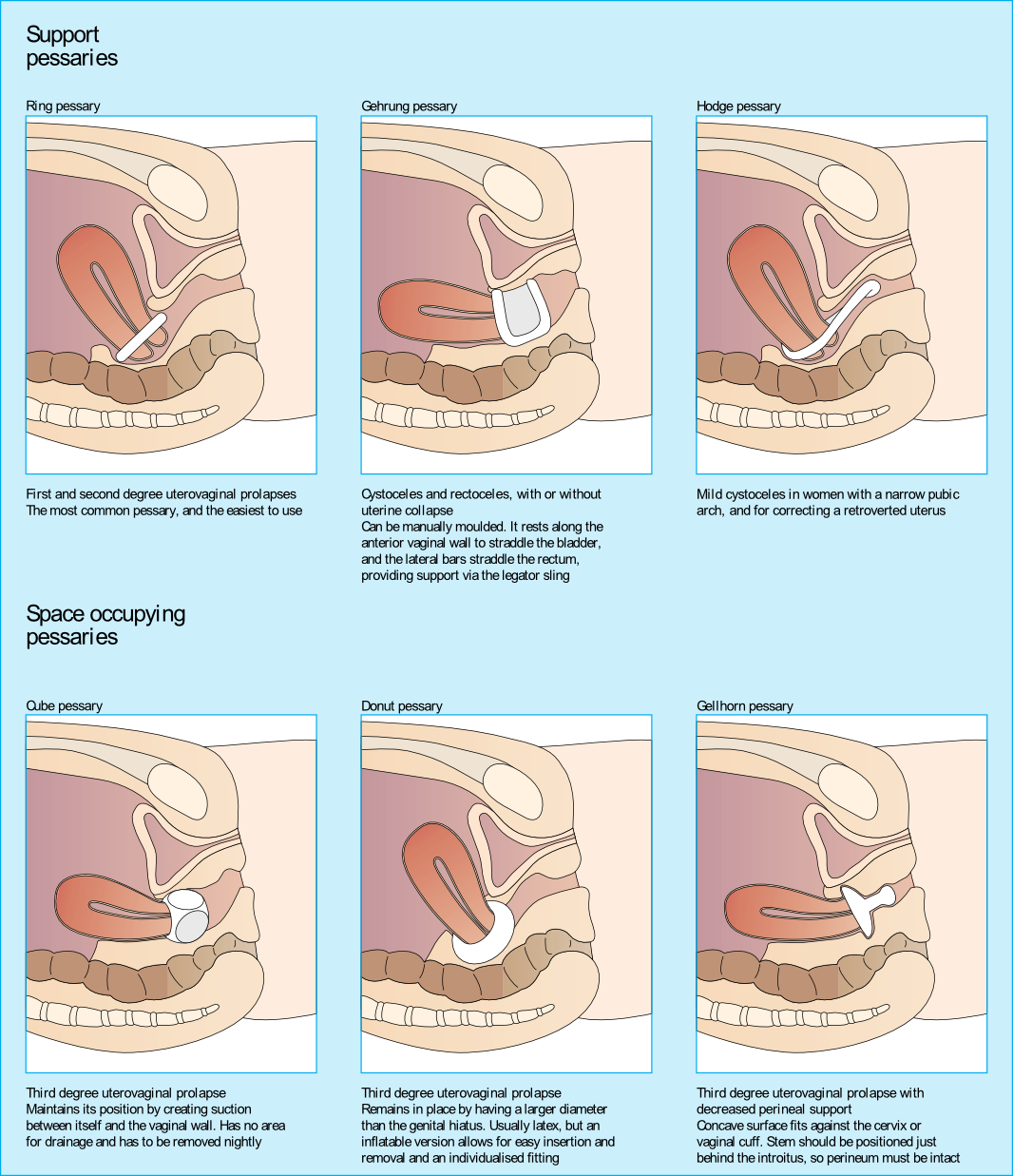
Upon entering her vaginal canal slowly, I start to move around and felt a ring of plastic. “Are you wearing a pessary?” I asked. “Pessary? Oh, yes, I forgot to tell you about that!”, she exclaimed. “How long have you been using it?” I asked. “About 3 years…” she answered.
I sent her back to the urogynecologist to get fit for another type of pessary as her muscle examination proved to be negative. Since that time, I have added the question “Do you wear a pessary?” as part of the constipation intake questions. Pessary use creates the ability for a patient to forgo or to extend their time for a surgical intervention due to pelvic organ prolapse.
Looking at the dynamics of the pessary, it may block bowel movement emptying. The recent study by Dengle, et al, published in the October 2018 in the International Urogynecological Journal confirms this anecdotal, clinical finding. The article, Defecatory Dysfunction and Other Clinical Variables Are Predictors of Pessary Discontinuation, looked at reasons for discontinuation of pessary use from April 2014 to January 2017 and did a retrospective chart review on a selected 1071 women. Incomplete defecation had the largest association with pessary discontinuation.
While there are over 20 sizes of pessaries on the market, patients will discontinue use without having a better conversation with their practitioner. From a PT perspective, when the patient comes in with bowel emptying issues, if no muscle dysfunction is found, it needs to be brought to the provider’s attention. Our role in educating the patient on the options that are available and creating this dialogue can prove to be very helpful in those suffering from pelvic organ prolapse and defecatory dysfunction.
Dengler, EG et al. "Defecatory dysfunction and other clinical variables are predictors of pessary discontinuation." Int Urogynecol J. 2018 Oct 20. doi: 10.1007/s00192-018-3777-1. https://www.ncbi.nlm.nih.gov/pubmed/30343377
The British author, John Donne, wrote, “No man is an island, entire of itself; every man is a piece of the continent.” In a similar idea, no neurological symptom is independent and isolated; every system has potential to impact the whole body. Neurogenic bladder should cue a clinician to check for neurogenic bowel and to assess the pelvic floor in order to get a complete map of what to address in treatment.
 Martinez, Neshatian, & Khavari (2016) reviewed literature on neurogenic bowel dysfunction (NBD) and neurogenic bladder in patients with neurological conditions such as multiple sclerosis (MS). Constipation and fecal incontinence can coexist with NBD, and a multifactorial bowel regimen is vital to conservative management in patients with neurological disorders. Nonpharmacological, pharmacological, and surgical approaches were reviewed in the article. Specific results for MS were reported only for transanal irrigation (TAI) and biofeedback. In TAI, fluid is used to stimulate the bowel and clean out stool from the rectum. A study showed 53% of the 30 patients with MS demonstrated a 50% or better improvement in bowel symptoms with TAI. In anorectal biofeedback, operant conditioning retrains motor and sensory responses via exercises guided by manometry. With biofeedback, a study showed 38% of patients had a beneficial impact with the intervention. The list of treatment approaches not specifically researched for MS patients in this review includes: dietary modifications, perianal/anorectal stimulation, abdominal massage, suppositories, oral medications such as stool softeners or prokinetic agents, sacral neuromodulation, antegrade continence enema, and colostomy.
Martinez, Neshatian, & Khavari (2016) reviewed literature on neurogenic bowel dysfunction (NBD) and neurogenic bladder in patients with neurological conditions such as multiple sclerosis (MS). Constipation and fecal incontinence can coexist with NBD, and a multifactorial bowel regimen is vital to conservative management in patients with neurological disorders. Nonpharmacological, pharmacological, and surgical approaches were reviewed in the article. Specific results for MS were reported only for transanal irrigation (TAI) and biofeedback. In TAI, fluid is used to stimulate the bowel and clean out stool from the rectum. A study showed 53% of the 30 patients with MS demonstrated a 50% or better improvement in bowel symptoms with TAI. In anorectal biofeedback, operant conditioning retrains motor and sensory responses via exercises guided by manometry. With biofeedback, a study showed 38% of patients had a beneficial impact with the intervention. The list of treatment approaches not specifically researched for MS patients in this review includes: dietary modifications, perianal/anorectal stimulation, abdominal massage, suppositories, oral medications such as stool softeners or prokinetic agents, sacral neuromodulation, antegrade continence enema, and colostomy.
Miletta, Bogliatto, & Bacchio (2017) presented a case study about management of sexual dysfunction, perineal pain, and elimination dysfunction in a 40 year old female with multiple sclerosis. She had been experiencing perineal pain for 5 months and had chronic MS symptoms of lower anourogenital dysfunction, including bladder retention and obstructed defecation syndrome. Physical therapy treatment included pelvic floor muscle training (primarily decreasing overactivity of pelvic muscles in this case), perineal massage, biofeedback, postural correction, global relaxation techniques, and a home self-training program. After 5 months of physical therapy, the woman had improved pelvic floor muscle contraction strength, resolution of pelvic floor muscle overactivity, increased sexual satisfaction (according to the Female Sexual Function Index score), a visual analog scale improvement of vulvar and perineal pain by 4 points, normalization of obstructed defecation syndrome, and decreased bladder retention symptoms. The authors concluded the variety of symptoms in MS require a multimodal approach for treatment, considering all the motor, autonomic, and cognitive impairments as well as side effects of medications that try to improve those symptoms. The quality of life of women with MS has potential to be improved significantly if pelvic floor disorders related to MS are addressed appropriately.
Ultimately, treating urinary dysfunction but avoiding bowel dysfunction does neurological patients a disservice. Systems are intertwined in a series of cause and effects throughout the body. The “Neurologic Conditions and the Pelvic Floor” course can expand your knowledge and understanding of how the symptoms of conditions such as multiple sclerosis can impact pelvic health and how we can better address the whole patient for optimal outcomes.
Martinez, L., Neshatian, L., & Khavari, R. (2016). Neurogenic Bowel Dysfunction in Patients with Neurogenic Bladder. Current Bladder Dysfunction Reports, 11(4), 334–340. http://doi.org/10.1007/s11884-016-0390-3
Miletta, M., Bogliatto, F., & Bacchio, L. (2017). Multidisciplinary Management of Sexual Dysfunction, Perineal Pain, and Elimination Dysfunction in a Woman with Multiple Sclerosis. International Journal of MS Care, 19(1), 25–28. http://doi.org/10.7224/1537-2073.2015-082