“Keep Calm and Treat Pain” is perhaps an affirmation for therapists when encountering patients suffering from pain, whether acute or chronic. The reality is this: treating pain is complicated. Treating pain has brought many a health care provider to his or her proverbial knees. It has also led us as a nation into the depths of the opioid epidemic which claimed over 165,000 lives between the years of 1999 and 2014 (Dowell & Haegerich, 2016). That number has swollen to over 200,000 in up-to-date calculations and according to the CDC, 42,000 human beings, not statistics, were killed by opioids in 2016 - a record.
 So why has treating pain eluded us as a nation? The answers are as complicated as treating pain itself. Which is why we as health care providers must seek out not simply alternatives, but the truth in the matter. Why are so many suffering? Why has chronic pain become the enormous beast that it has become? What might we do differently, collectively, and how might we examine this issue through a holistic mindset?
So why has treating pain eluded us as a nation? The answers are as complicated as treating pain itself. Which is why we as health care providers must seek out not simply alternatives, but the truth in the matter. Why are so many suffering? Why has chronic pain become the enormous beast that it has become? What might we do differently, collectively, and how might we examine this issue through a holistic mindset?
In just a few weeks, I have the privilege of teaching amongst 10 physical therapy professionals and one physician from around the nation who with coordinated efforts created a landmark pre-conference course at CSM in New Orleans through the Orthopaedic Section of the APTA. Included in the 11 are myself and another Herman & Wallace instructor Carolyn McManus, PT, MS, MA who teaches “Mindfulness Based Pain Treatment” through the Institute.
The CSM pre-conference course title is “Keep Calm and Treat Pain” representing a necessary effort to provide the clinician with ideas and inspiration for helping the profession as a whole treat pain with an integrative approach.
“Pain and Nutrition: Building Resilience Through Nourishment” is the section I look forward to sharing. It will introduce concepts we can leverage to allow us confidence in seeking alternate ways of taming this beast which is chronic pain - ways which can enhance health and well-being of our clients in pelvic rehabilitation. We must not be passive observers of the opioid epidemic. We must come to terms with the fact that our nations go-to tool for treating pain unfortunately causes side-effects which can and does include loss of life. We can do better. And we will.
While the CSM pre-conference course will give you a taste of the nutrition concepts available to you, it is a mere tip of the nourishment iceberg. I continue my passion and mission with the two-day course titled “Nutrition Perspectives for the Pelvic Rehab Therapist”, an experience that can elevate your conversations with clients. It will pave a path of understanding for the provider, allowing us to share options, understanding, and hope. “Nutrition Perspectives for the Pelvic Rehab Therapist is coming next to Maywood, IL March 3 & 4, 2018. I welcome you to join me.
APTA CSM: https://apta.expoplanner.com/index.cfm?do=expomap.sess&event_id=27&session_id=13763. Accessed January 8, 2018.
CDC: https://www.cdc.gov/drugoverdose/index.html. Accessed January 8, 2018.
Dowell, D., & Haegerich, T. M. (2016). Using the CDC Guideline and Tools for Opioid Prescribing in Patients with Chronic Pain. Am Fam Physician, 93(12), 970-972.
Lerner, A., Neidhofer, S., & Matthias, T. (2017). The Gut Microbiome Feelings of the Brain: A Perspective for Non-Microbiologists. Microorganisms, 5(4). doi:10.3390/microorganisms5040066
Murthy, V. H. (2016). Ending the Opioid Epidemic - A Call to Action. N Engl J Med, 375(25), 2413-2415. doi:10.1056/NEJMp1612578
The new year is here and with it, lots of motivational posting about exercise and weight loss…but how is this desire for ‘new year, new you’ affecting peri-menopausal women with urinary dysfunction? It has been established that the lower urinary tract is sensitive to the effects of estrogen, sharing a common embryological origin with the female genital tract, the urogenital sinus. Urge urinary incontinence is more prevalent after the menopause, and the peak prevalence of stress incontinence occurs around the time of the menopause (Quinn et al 2009). Zhu et al looked at the risk factors for urinary incontinence in women and found that some of the main contributors include peri/post-menopausal status, constipation and central obesity (women's waist circumference, >/=80 cm) along with vaginal delivery/multiparity.
 Could weight loss directly impact urinary incontinence in menopausal women? In a word – yes. ‘Weight reduction is an effective treatment for overweight and obese women with UI. Weight loss of 5% to 10% has an efficacy similar to that of other nonsurgical treatments and should be considered a first line therapy for incontinence’ (Subak et al 2005) But do these benefits last? Again – yes! ‘Weight loss intervention reduced the frequency of stress incontinence episodes through 12 months and improved patient satisfaction with changes in incontinence through 18 months. Improving weight loss maintenance may provide longer term benefits for urinary incontinence.’ (Wing et al 2010)
Could weight loss directly impact urinary incontinence in menopausal women? In a word – yes. ‘Weight reduction is an effective treatment for overweight and obese women with UI. Weight loss of 5% to 10% has an efficacy similar to that of other nonsurgical treatments and should be considered a first line therapy for incontinence’ (Subak et al 2005) But do these benefits last? Again – yes! ‘Weight loss intervention reduced the frequency of stress incontinence episodes through 12 months and improved patient satisfaction with changes in incontinence through 18 months. Improving weight loss maintenance may provide longer term benefits for urinary incontinence.’ (Wing et al 2010)
The other major health issues facing women at midlife include an increased risk for cardiovascular disease, Type 2 Diabetes and Bone Health problems – all of which are responsive to lifestyle interventions, particularly exercise and stress management. In their paper looking at lifestyle weight loss interventions, Franz et al found that ‘…a weight loss of >5% appears necessary for beneficial effects on HbA1c, lipids, and blood pressure. Achieving this level of weight loss requires intense interventions, including energy restriction, regular physical activity, and frequent contact with health professionals’. 5% weight loss is the same amount of weight loss necessary to provide significant benefits for urinary incontinence at midlife.
Successful weight management depends on nutritional intake, exercise and psychosocial considerations such as stress management, but for the menopausal woman, hormonal balance can also have an effect on not only bladder and bowel dysfunction but changing metabolic rates, thyroid issues and altered weight distribution patterns. As pelvic rehab therapists, we are all aware that pelvic health issues can be a barrier to exercise participation but sensitive awareness of the other particular challenges facing midlife women can make the difference in developing a beneficial therapeutic alliance and a journey back to optimal health. If you would like to explore the topics surrounding optimal health at menopause, why not join me in California in February?
Climacteric. 2009 Apr;12(2):106-13. ‘The effects of hormones on urinary incontinence in postmenopausal women.’ Quinn SD, Domoney C. Menopause. 2009 Jul-Aug;16(4):831-6. The epidemiological study of women with urinary incontinence and risk factors for stress urinary incontinence in China’ Zhu L, Lang J, Liu C, Han S, Huang J, Li X. J Urol. 2005 Jul;174(1):190-5. Weight loss: a novel and effective treatment for urinary incontinence’ Subak LL, Whitcomb E, Shen H, Saxton J, Vittinghoff E, Brown JS. J Urol. 2010 Sep;184(3):1005-10. Effect of weight loss on urinary incontinence in overweight and obese women: results at 12 and 18 months Wing RR, West DS, Grady D, Creasman JM, Richter HE, Myers D, Burgio KL, Franklin F, Gorin AA, Vittinghoff E, Macer J, Kusek JW, Subak LL; Program to Reduce Incontinence by Diet and Exercise Group. J Acad Nutr Diet. 2015 Sep;115(9):1447-63. doi: 10.1016/j.jand.2015.02.031. Epub 2015 Apr 29. Lifestyle weight-loss intervention outcomes in overweight and obese adults with type 2 diabetes: a systematic review and meta-analysis of randomized clinical trials. Franz MJ, Boucher JL, Rutten-Ramos S, VanWormer JJ. Lean, M, & Lara, J & O Hill, J (2007) Strategies for preventing obesity. In: Sattar, N & Lean, M (eds.) ABC of Obesity. Oxford, Blackwell Publishing.
Mindful eating requires slowing down and paying attention to the present moment experience of eating. Rather than mindlessly put food into your mouth and not really taste what you’re eating, you deliberately notice the appearance, smell, texture and taste of the food and pay attention to your thoughts, feelings, and physical sensations. Eating mindfully can interrupt habitual eating behaviors and promote greater self-regulation of food choices.1 Warren and colleagues conclude mindful eating has the potential to help address maladaptive eating behaviors and the difficulties many face with controlling food intake.2
 Although mindful breathing, body scan and movement are the core skills I teach patients with persistent pain, I introduce mindful eating as another strategy to cultivate present moment awareness. Patients can have surprising shifts in their relationship to food and frequently comment, “If I ate more mindfully, I would enjoy my food more and eat less!”
Although mindful breathing, body scan and movement are the core skills I teach patients with persistent pain, I introduce mindful eating as another strategy to cultivate present moment awareness. Patients can have surprising shifts in their relationship to food and frequently comment, “If I ate more mindfully, I would enjoy my food more and eat less!”
Lucie Khadduri, PT, DPT, PRPC clinician and Adjunct Professor at the University of Puget Sound School of Physical Therapy, took my course last spring and describes her patient’s experience with mindful eating:
I have been meaning to email for some time to thank you for the April 2017 course on Mindfulness for Rehab Professionals. Your class really impacted my daily PT practice in a positive way. I wanted to share with you one story in particular to illustrate the power that these new tools you have given me have helped others.
I have this male patient who is about 35 years old who struggled with chronic constipation, bloating and anxiety related to his intense fecal urges that were then followed by an inability to defecate. When he started PT, he had just left his job and took a job working from home just so that he could have consistent, stress free bathroom access.
I spoke to him about diaphragmatic breathing and mindfulness and its impact on the autonomic nervous system. What helped him the most, though, was the mindful eating exercise. He has since started applying these concepts to when he eats. He told me on his discharge visit that in the past, he would eat 4 slices of pizza very quickly, without thinking about it and then have horrible pain afterward. Now, he says it is easy to eat 1 slice and have a salad not because he knows salad is better for him, but because his mouth and mind crave different textures and colors in his food. Mindful eating gave him the ability to slow down, focus on the physical sensations of eating and he found that this has changed his relationship with food. As a result, his constipation is much better managed and his anxiety and stress are much better.
Thanks again for an excellent class. I often encourage patients to go to your website for your free 10 minute meditations.
Thank you, Lucie, for sharing this story. It reflects one of the many ways patients benefit from training in mindful awareness. I look forward to introducing colleagues to mindful eating and additional experiential mindful exercises and current research in my upcoming class, Mindfulness-Based Pain Treatment, at Loyola University Stritch School of Medicine, Maywood, Il, September 30 and October 1.
1. Miller CK. Mindful eating with diabetes. Diabetes Spectr. 2017 May;30(2):89-94.
2. Warren JM, Smith N, Ashwell M. A structured literature review on the role of mindfulness, mindful eating and intuitive eating in changing eating behaviors: effectiveness and associated potential mechanisms. Nutr Res Rev. 2017 Jul 18:1 – 12.
Image courtesy of California Institute of Technology
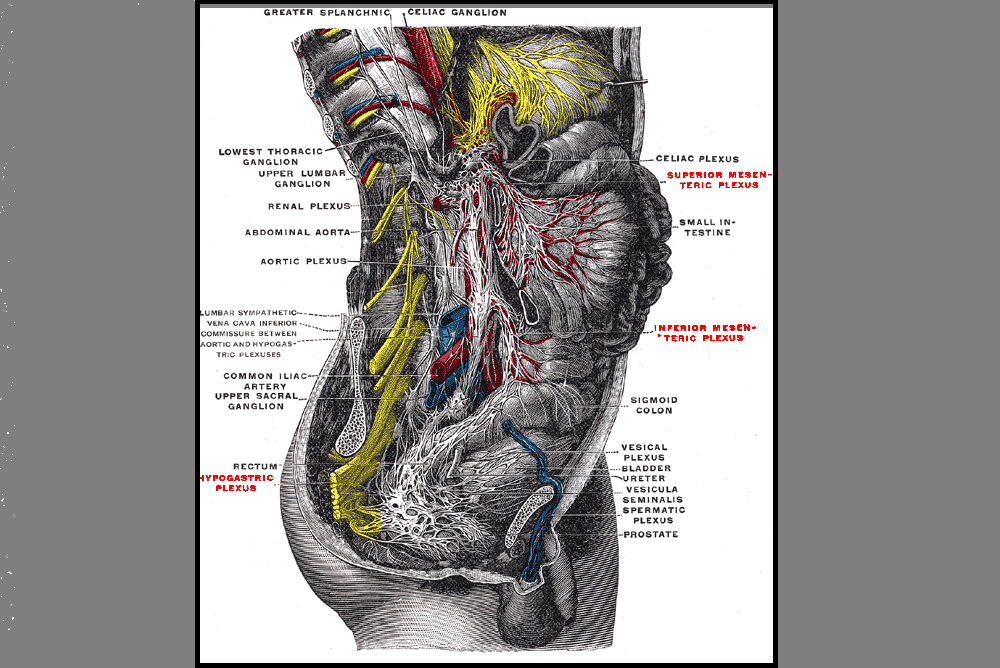
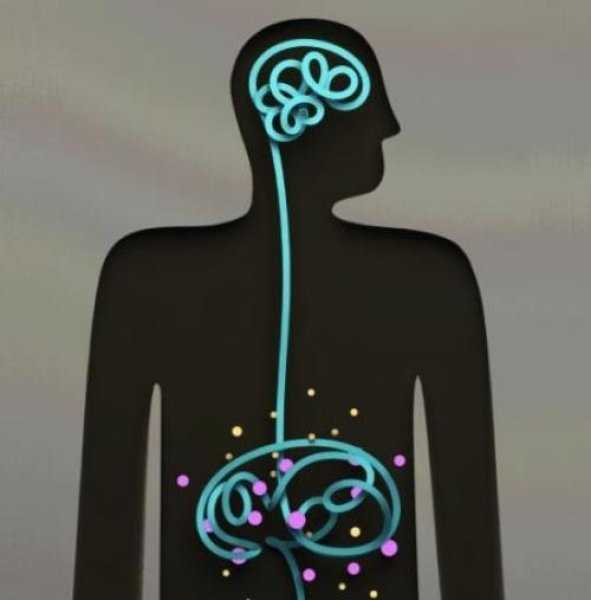
Anxiety and depression are frequently encountered co-morbidities in the clients we serve in pelvic rehabilitation. This observation several years ago in clinical practice is one of many that prompted me down the path of exploring the connection between the gut, the brain, and overall health. In answering the question about these connections, I discovered many nutritionally related truths that are being rapidly elucidated in the literature.
A recent study by Sandhu, et.al. (2017) examines the role of the gut microbiota on the health of the brain and it’s influence on anxiety and depression. The title of the study, “Feeding the microbiota-gut-brain axis: diet, microbiome, and neuropsychiatry” gives us pause to consider the impact of our diets on this axis and in turn, on the health of our nervous system. The authors state:
It is diet composition and nutritional status that has been repeatedly been shown to be one of the most critical modifiable factors regulating the gut microbiota at different time points across the lifespan and under various health conditions.
With diet and nutritional status being the most critical modifiable factors in the health of this system, it becomes our responsibility to seek to understand this system and its influencing factors. We need to learn how to nourish the microbiota-gut-brain axis.
While anxiety and depression are common co-morbidities we encounter, we also commonly detect imbalance between the sympathetic and parasympathetic nervous system in our patients leading to, for example, pelvic floor muscle tension. In light of this study we must first and foremost ask: what is the microbiota? How can it influence our nervous system? How does this correlate to anxiety and depression? The answers to these questions provide clinical insight with far-reaching impact. We also consider: which circumstances disrupt the health of this system and which improve it? Finally, could understanding of this axis, among other nutritional correlates, provide a novel approach to bowel dysfunction, bladder dysfunction, chronic pelvic pain?
Be a part of the paradigm shift to integrative understanding as we explore these and many other burning questions. Please join us for insightful discussion in White Plains, NY March 31-April 1, 2017 for our next offering of Nutrition Perspectives for the Pelvic Rehab Therapist.
Sandhu, K. V., Sherwin, E., Schellekens, H., Stanton, C., Dinan, T. G., & Cryan, J. F. (2017). Feeding the microbiota-gut-brain axis: diet, microbiome, and neuropsychiatry. Transl Res, 179, 223-244. doi:10.1016/j.trsl.2016.10.002
Honestly, I have never noticed Curcumin on any of my patients’ lists of pharmaceuticals or supplements, but I will be certain to look for it now. Curcumin is the fat-soluble molecule that gives turmeric its yellow pigment, and it is best absorbed with the addition of black pepper extract. Patients often complain non-steroidal anti-inflammatory medicines (NSAIDs) tear apart their stomachs, so newer studies showing positive results with the use of an herb sound promising, even for pelvic health.
 A 2015 study by Kim et al. researched the inhibitory effect of curcumin on benign prostatic hyperplasia induced by testosterone in a rat model. Benign prostatic hyperplasia (BPH) is common among men and has a negative impact on the urinary tract of older males. Steroid 5-alpha reductase converts testosterone into dihydrotestosterone (DHT), and this increases as men age and may have negative effects on the prostate gland. Because of the side effects of conventional drugs (like finasteride) to inhibit steroid 5-alpha reductase, the authors wanted to determine if curcumin could play a protective role in BPH. They divided 8 rats into 4 groups after removal of testicles: 1) normal, 2) BPH testosterone induced subcutaneously, 3) daily curcumin (50mg/kg orally), and 4) daily finasteride (1mg/kg orally). The group receiving curcumin had significantly lower prostate weight and volume than the testosterone induced BPH group, and curcumin decreased the expression of growth factors in prostate tissue. The authors conclude curcumin may be a useful herb in inhibiting the development of BPH with fewer side effects than conventional drugs.
A 2015 study by Kim et al. researched the inhibitory effect of curcumin on benign prostatic hyperplasia induced by testosterone in a rat model. Benign prostatic hyperplasia (BPH) is common among men and has a negative impact on the urinary tract of older males. Steroid 5-alpha reductase converts testosterone into dihydrotestosterone (DHT), and this increases as men age and may have negative effects on the prostate gland. Because of the side effects of conventional drugs (like finasteride) to inhibit steroid 5-alpha reductase, the authors wanted to determine if curcumin could play a protective role in BPH. They divided 8 rats into 4 groups after removal of testicles: 1) normal, 2) BPH testosterone induced subcutaneously, 3) daily curcumin (50mg/kg orally), and 4) daily finasteride (1mg/kg orally). The group receiving curcumin had significantly lower prostate weight and volume than the testosterone induced BPH group, and curcumin decreased the expression of growth factors in prostate tissue. The authors conclude curcumin may be a useful herb in inhibiting the development of BPH with fewer side effects than conventional drugs.
In the urology realm, Cosentino et al.2016 explored the anti-inflammatory effects of a product called Killox®, a supplement with curcumin, resveratrol, N-acetylcysteine (NAC) and zinc. When benign prostatic hyperplasia (BPH) is not treated with drugs, a surgical intervention can be executed called a transurethral resection of the prostate (TURP); or, for bladder cancers, a transurethral resection of the bladder (TURB) can be performed. Either surgery generally requires administration of NSAIDs post-operatively for inflammation, urinary burning, or bladder spasms or to prevent later complications such as urethral stricture or sclerosis of the bladder neck. This open controlled trial involved Killox® tablet administration to 40 TURP patients twice a day for 20 days, to 10 TURB patients twice a day for 10 days and to 30 BPH patients who were not suited for surgical intervention once a day for 60 days. The control group received nothing for 1 week post-surgery, and 52.5% of TURP and 40% of TURB patients required NSAIDs to treat burning and inflammation the following 7 days. None of the Killox® treatment groups had post-operative or late complications except one, and none suffered epigastric pain like those using NSAIDs. The authors concluded Killox® had significant positive anti-inflammatory and analgesic effects on the patients and could be used as a safe alternative to NSAIDs by physicians.
Although “just” an herb, the use of curcumin should be supervised by a healthcare professional who understands proper dosage and any possible contraindications for a particular individual. The curcumin needs to be in a form that can be easily digested and used effectively by the body. Ultimately, it is exciting to learn about an alternative to gut-wrenching NSAIDs, making curcumin a noteworthy anti-inflammatory option for patients.
Nutrition plays an important part in patient wellness and rehabilitation. There are many reasons to consider diet when designing treatment regimens and you can learn all about them in Megan Pribyl's Nutrition Perspectives for the Pelvic Rehab Therapist course. Your next chance to take this course is March 31 - April 1, 2017 in White Plains, NY. Don't miss out!
Kim, S. K., Seok, H., Park, H. J., Jeon, H. S., Kang, S. W., Lee, B.-C., … Chung, J.-H. (2015). Inhibitory effect of curcumin on testosterone induced benign prostatic hyperplasia rat model. BMC Complementary and Alternative Medicine,15, 380. http://doi.org/10.1186/s12906-015-0825-y
Cosentino, V., Fratter, A., Cosentino M. (2016). Anti-inflammatory effects exerted by Killox®, an innovative formulation of food supplement with curcumin, in urology. Eur Rev Med Pharmacol Sci. 20: 7, 1390-1398. http://www.ncbi.nlm.nih.gov/pubmed/27097964#
We are all familiar with the old saying, “You are what you eat.” A functional medicine lecture I attended recently at the Cleveland Clinic explained how chronic pain can be a result of how the body fails to process the foods we eat. Patients who just don’t seem to get better despite our skilled intervention make us wonder if something systemic is fueling inflammation. Even symptoms of vulvodynia, an idiopathic dysfunction affecting 4-16% of women, have been shown to correlate to diet.
In a single case study of a 28 year old female athlete in Integrative Medicine (Drummond et al., 2016), vulvodynia and irritable bowel syndrome (IBS) were addressed with an elimination diet. After being treated by a pelvic floor specialist for 7 months for vulvodynia, the patient was referred out for a nutrition consultation. Physical therapy was continued during the vegetarian elimination diet. In the patient’s first follow up 2 weeks after starting eliminating meat, dairy, soy, grains, peanuts, corn, sugar/artificial sweeteners, she no longer had vulvodynia. The nutrition specialist had her add specific foods every 2 weeks and watched for symptoms. Soy, goat dairy, and gluten all caused flare ups of her vulvodynia throughout the process. Eliminating those items and supplementing with magnesium, vitamin D3, probiotics, vitamin B12, and omega-3 allowed the patient to be symptom free of both vulvodynia and IBS for 6 months post-treatment.
 On the more scientific end of research, Vicki Ratner published a commentary called “Mast cell activitation syndrome” in 2015. She described how mast cells appear close to blood vessels and nerves, and they release inflammatory mediators when degranulated; however, mast cell activation syndrome (MCAS) involves mast cells that do not get degranulated properly and affect specific organs like the bladder. She proposed measuring the number of mast cells and inflammatory mediators in urine for more expedient diagnosis of interstitial cystisis and bladder pain syndrome.
On the more scientific end of research, Vicki Ratner published a commentary called “Mast cell activitation syndrome” in 2015. She described how mast cells appear close to blood vessels and nerves, and they release inflammatory mediators when degranulated; however, mast cell activation syndrome (MCAS) involves mast cells that do not get degranulated properly and affect specific organs like the bladder. She proposed measuring the number of mast cells and inflammatory mediators in urine for more expedient diagnosis of interstitial cystisis and bladder pain syndrome.
Sigrid Regauer’s correspondence to Ratner’s article followed in 2016 relating MCAS to bladder pain syndrome (BPS), interstitial cystitis (IC), and vulvodynia. He described vulvodynia as a pain syndrome with excessive mast cells and sensory nerve hyperinnervation, often found with BPS and IC. The vulvodynia patients had mast cell hyperplasia, most of which were degranulated, and 70% of the patients had comorbidities due to mast cell activation such as food allergies, histamine intolerance, infections, and fibromyalgia.
Considering the association between mast cells and acute inflammatory responses and how mast cells release proinflammatory mediators, it makes sense that dysfunctions such as vulvodynia as well as IC and BPS can result from an excessive amount and dysfunctional granulation of mast cells. Enhanced activation of mast cells causes histamine release, stimulating peripheral pain neurotransmitters (Fariello & Moldwin 2015). If medication and therapy do not solve a patient’s pain, perhaps eliminating the consumption of inflammatory foods could positively affect the body on a cellular level and relieve irritating symptoms of vulvodynia. Pardon the parody, but patients on the brink of being “insane in the brain” from vulvodynia will likely try anything to resolve being “inflamed in the membrane.”
Drummond, J., Ford, D., Daniel, S., & Meyerink, T. (2016). Vulvodynia and Irritable Bowel Syndrome Treated With an Elimination Diet: A Case Report.Integrative Medicine: A Clinician’s Journal, 15(4), 42–47.
Ratner, V. (2015). Mast cell activation syndrome. Translational Andrology and Urology, 4(5), 587–588. http://doi.org/10.3978/j.issn.2223-4683.2015.09.03
Regauer, S. (2016). Mast cell activation syndrome in pain syndromes bladder pain syndrome/interstitial cystitis and vulvodynia. Translational Andrology and Urology, 5(3), 396–397. http://doi.org/10.21037/tau.2016.03.12
Fariello, J. Y., & Moldwin, R. M. (2015). Similarities between interstitial cystitis/bladder pain syndrome and vulvodynia: implications for patient management. Translational Andrology and Urology, 4(6), 643–652. http://doi.org/10.3978/j.issn.2223-4683.2015.10.09
Appropriate sun exposure and/or daily supplements provide our bodies with sufficient amounts of Vitamin D. I would venture to guess almost every one of the patients I treated in Seattle had a deficiency of Vitamin D if they were not taking a supplement. Running outside year round has always kept my skin slightly tan and my levels of Vitamin D healthy; however, when I was pregnant in the Pacific Northwest, I had to supplement my diet with Vitamin D, which was a first for this East Coast beach girl. The benefit of Vitamin D has spread beyond just bone health, with studies showing its impact on pelvic floor function.
Parker-Autry et al., (2012) published a study discerning the Vitamin D levels in women who already presented with pelvic floor dysfunction versus “normal” gynecological patients. The retrospective study involved a chart review of 394 women who completed the Colorectal Anal Distress Inventory (CRADI)-8 and the Incontinence Impact Questionnaire (IIQ-7). These women all had a total serum 25-hydroxy Vitamin D [25(OH)D] drawn within one year of their gynecological visit. The authors defined a serum 25(OH)D of <15ng/ml as Vitamin D deficient, between 15-29ng/ml as Vitamin D insufficient, and >30ng/ml as Vitamin D sufficient. In the pelvic floor disorder group comprised of 268 women, 51% were found Vitamin D insufficient, 13% of whom were deficient. The CRADI-8 and IIQ-7 scores were noted as higher among the Vitamin D insufficient women. Overall, the mean 25(OH)D levels in the women without pelvic floor issues were higher than those who presented with pelvic floor disorder symptoms.
Another case-control study in 2014 by Parker-Autry et al., focused on the association between Vitamin D deficiency and fecal incontinence. They considered 31 women with fecal incontinence versus a control group of 81 women without any pelvic floor symptoms, looking at serum Vitamin D levels. The women with fecal incontinence had a mean serum Vitamin D level of 29.2±12.3 ng/ml (insufficient/deficient), while the control group had a higher mean level of 35±14.1 ng/ml (sufficient). The women completed the Modified Manchester Health Questionnaire and the Fecal Incontinence Severity Index, and women with deficient Vitamin D scored higher on the questionnaire, indicating fecal incontinence as a burden on quality of life. The severity scores were higher for Vitamin D deficient women, but there was not a statistically significant difference between the groups. Once again, the pelvic floor disorder and Vitamin D deficiency correlation prevailed in this study.
An even more recent study looked at postmenopausal women and Vitamin D deficiency (Navaneethan et al., 2015). This prospective case control study involved 120 postmenopausal women, 51 of whom had pelvic floor disorders. The serum 25-hydroxy Vitamin D levels were obtained, and the results revealed a deficiency in those women with pelvic floor dysfunction. Vitamin D levels were found to be significantly lower in women who were 5 years or more into menopause. Overall, Vitamin D was deemed a worthy factor to consider in the pelvic floor disorder population as well as in postmenopausal women.
Taking time to talk to patients about their lifestyle, daily supplements, and diet can often shed light on their ability to benefit from our treatments. If a Vitamin D deficiency sounds possible, discuss current research with them and suggest they get their serum Vitamin D levels checked. Don’t underestimate the power of a little sunshine – it just might have a positive impact on pelvic floor health.
Parker-Autry, C. Y., Markland, A. D., Ballard, A. C., Downs-Gunn, D., & Richter, H. E. (2012). Vitamin D Status in Women with Pelvic Floor Disorder Symptoms. International Urogynecology Journal, 23(12), 1699–1705. http://doi.org/10.1007/s00192-012-1700-8
Parker-Autry, C. Y., Gleason, J. L., Griffin, R. L., Markland, A., & Richter, H. E. (2014). VITAMIN D DEFICIENCY IS ASSOCIATED WITH INCREASED FECAL INCONTINENCE SYMPTOMS. International Urogynecology Journal, 25(11), 1483–1489. http://doi.org/10.1007/s00192-014-2389-7
Navaneethan, P. R., Kekre, A., Jacob, K. S., & Varghese, L. (2015). Vitamin D deficiency in postmenopausal women with pelvic floor disorders. Journal of Mid-Life Health, 6(2), 66–69. http://doi.org/10.4103/0976-7800.158948
The American Society of Clinical Oncology convened their 2016 annual meeting over the weekend, and several of the presentations suggest new methods of preventing breast cancer recurrence.
Extended Hormone Therapy Reduces Recurrence of Breast Cancer
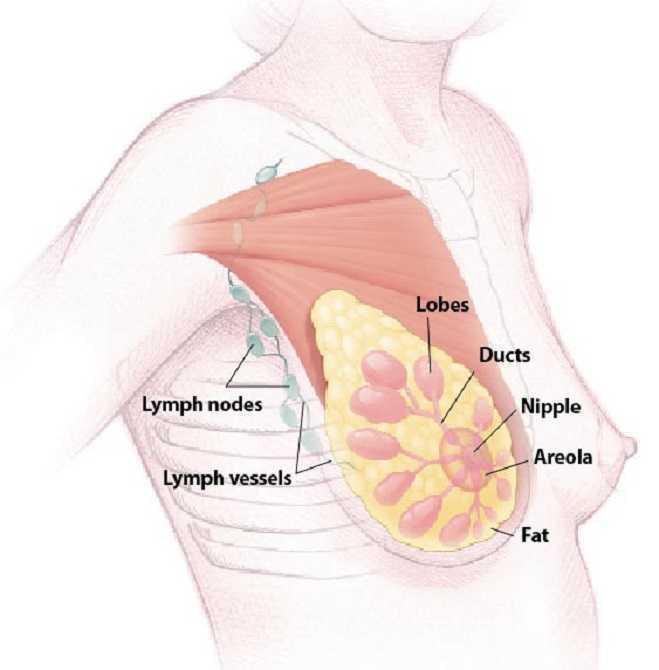 Breast cancer patients who are treated with aromatase inhibitor therapy are generally prescribed the the estrogen drugs for a five year course. A new study has suggested that by doubling the length of hormone therapy, the recurrence rate for breast cancer survivors drops by 34%. The study included 1,918 women who underwent five years of hormone therapy with the drug letrozole. After five years, half of the group switched to a placebo while the other half were given an additional five year treatment.
Breast cancer patients who are treated with aromatase inhibitor therapy are generally prescribed the the estrogen drugs for a five year course. A new study has suggested that by doubling the length of hormone therapy, the recurrence rate for breast cancer survivors drops by 34%. The study included 1,918 women who underwent five years of hormone therapy with the drug letrozole. After five years, half of the group switched to a placebo while the other half were given an additional five year treatment.
Drug Used to Treat Type 2 Diabetes May Increase Breast Cancer Survivability
The Univerisity of Pennsylvania School of Medicine has published results from two recent studies which document the effects of Metformin, a drug commonly used to treat type 2 diabetes, on breast cancer and endometrial hyperplasia. The study tracked outcomes for 1,215 patients who were diagnosed and surgically treated for breast cancer. Patients who began to use metformin after their diagnosis were found to have a 50% higher survivability rate than those who did not use metformin.
The timing of metformin use is extremely important when it comes to breast cancer survivability rates. The study also found that patients who used metformin prior to their diagnosis were more than twice as likely to die than those who never used the drug.
Research Suggests a "Mediterranian Diet" May Reduce Breast Cancer Recurrence
A study has indicated that a diet rich in vegetables, fish, and olive oil may decrease the odds of a breast cancer survivor experiencing a relapse or recurrence of their cancer. The study tracked 300 women with early-stage cancer and found that those who ate a normal diet were more likely to experience a breast cancer recurrence. The findings build upon previous research which indicated that a Mediterranean diet, and especially extra virgin olive oil, could reduce breast cancer risk by 68%.
Want to Learn More?
Susannah Haarmann, PT, CLT, WCS is the author and instructor of Physical Therapy Treatment for the Breast Oncology Patient, a course offered through the Herman & Wallace Institute. This continuing education course for medical practitioners offers a rehabilitation perspective for providers who work with oncology rehabilitation patients. Join Dr. Haarmann this in Stockton, CA on September 24-25 to learn evaluation and treatment techniques necessary to make an outpatient therapist an essential member of any oncology team.
1) Paul E. Goss, et al. J Clin Oncol 34, 2016 (suppl; abstr LBA1)
https://www.asco.org/about-asco/press-center/news-releases/ten-years-hormone-therapy-reduces-breast-cancer-recurrence
2) Yun Rose Li. University of Pennsylvania, American Society of Clinical Oncology Annual Meeting 2016
http://www.eurekalert.org/pub_releases/2016-06/uops-ddm060316.php
3) http://www.scienceworldreport.com/articles/41404/20160606/mediterranean-diet-prevent-breast-cancer-recurring.htm
When a 472 pound gentleman recently arrived for an evaluation for low back pain, he came to the clinic for me to help him, not deride him about his weight (which he complained all his doctors have already done). He claimed he had lost 120 pounds but gained back 50, and his low back was extremely painful with transitional movements and daily function. Undoubtedly, this man’s body was a battlefield for inflammation, and no matter how much manual therapy or exercise I implemented, nutrition education seemed vital. Instead of just chatting about baseball or the weather, competently sharing what we’ve studied and learned in continuing education courses is warranted in our practice.
 In a 2016 review Klek reveals the most current evidence regarding Omega-3 Fatty Acids in nutrition delivered intravenously. Although physical therapists do not decide the ingredients for patients’ parenteral nutrition, the article thoroughly explains the essential benefits of fatty acids. Aside from being important structural components of cell membranes and precursors of prostaglandins and cholesterol, fatty acids regulate gene expression and adjust pathways of cells regarding inflammation and cell-mediated immune responses. Ultimately, fatty acids modulate metabolic processes in the body, whether locally, in a particular region, or at remote sites. Omega-3 fatty acids have been shown to inhibit synthesis of triglycerides by the liver, prevent cardiovascular disease, reduce cancerous cell growth, and even affect the development of rheumatoid arthritis and Chrohn’s disease. This article not only sheds light on parenteral nutrition for post-surgical, oncology, critically ill, and even pediatric patients but also educates the healthcare professional on the impact fatty acids have on the patients we treat.
In a 2016 review Klek reveals the most current evidence regarding Omega-3 Fatty Acids in nutrition delivered intravenously. Although physical therapists do not decide the ingredients for patients’ parenteral nutrition, the article thoroughly explains the essential benefits of fatty acids. Aside from being important structural components of cell membranes and precursors of prostaglandins and cholesterol, fatty acids regulate gene expression and adjust pathways of cells regarding inflammation and cell-mediated immune responses. Ultimately, fatty acids modulate metabolic processes in the body, whether locally, in a particular region, or at remote sites. Omega-3 fatty acids have been shown to inhibit synthesis of triglycerides by the liver, prevent cardiovascular disease, reduce cancerous cell growth, and even affect the development of rheumatoid arthritis and Chrohn’s disease. This article not only sheds light on parenteral nutrition for post-surgical, oncology, critically ill, and even pediatric patients but also educates the healthcare professional on the impact fatty acids have on the patients we treat.
In 2015, Haghiac et al. performed a randomized double-blind controlled clinical trial to determine if Omega-3 fatty acid supplementation could reduce inflammation in pregnant woman who are obese. Although the study began with 36 subjects in each group, only 24 women in the experimental group receiving 4 capsules a day of Omega-3 fatty acid (total of 2000mg) and 25 of the women taking 4 placebo capsules a day completed the supplementation over the 25 weeks up until delivery. The authors referenced the findings that low grade inflammation becomes exacerbated in obese pregnant women. While an excess of Omega-6 fatty acids practically promotes inflammation via eicosanoid (hormone) production, a healthy balance of Omega-3 fatty acids lessens inflammatory and immunosuppressive eicosanoid production. This study demonstrated an improvement in inflammation in the women who took the Omega-3 fatty acid as evidenced by a decrease in the expression of inflammatory genes in adipose tissue and placenta as well as reduced plasma C-reactive protein (CRP) at delivery.
Being able to control or reduce inflammation on a cellular level through nutrition could promote an exciting cycle of positive events for obese patients. Decreased inflammation in the body could decrease pain, which could allow and even promote increased activity and likely boost metabolism to equip them to battle obesity. The “Nutrition Perspectives for the Pelvic Rehab Therapist” course should spark the interest of any therapist wanting to guide patients not only on movement and function but also on the appropriate nutrition that best facilitates the body’s ability to heal and perform.
To learn more about nutrition and it's effects on pelvic rehabilitation, check out Nutrition Perspectives for the Pelvic Rehab Therapist this month in Lodi, CA.
Klek, S. (2016). Omega-3 Fatty Acids in Modern Parenteral Nutrition: A Review of the Current Evidence. Journal of Clinical Medicine, 5(3), 34. http://doi.org/10.3390/jcm5030034
Haghiac, M., Yang, X., Presley, L., Smith, S., Dettelback, S., Minium, J., … Hauguel-de Mouzon, S. (2015). Dietary Omega-3 Fatty Acid Supplementation Reduces Inflammation in Obese Pregnant Women: A Randomized Double-Blind Controlled Clinical Trial. PLoS ONE, 10(9), e0137309. http://doi.org/10.1371/journal.pone.0137309
In Megan Pribyl’s course on Nutrition Perspectives for the Pelvic Rehab Therapist, she discusses a wide variety of useful topics specific to nutrition and pelvic health. In her lecture on “Nutritional Homeostasis”, Megan counsels against missing an underlying eating disorder when working with a patient who has bowel issues. Work by Abraham and Kellow (2013) is cited, and in their article published in BMC Gastroenterology, the authors concur that many patients who have functional gastrointestinal complaints may also have disordered eating. How then, can we tell these patients apart, and get patients the most appropriate care? First let’s look at their research.
 Patients who were admitted to a specialty unit for those with eating disorders in Australia were studied and were found to have conditions such as anorexia nervosa, bulimia nervosa, polycystic ovarian syndrome, treated celiac disease, and treated bipolar depression. All of the 185 patients completed the Rome II Modular Questionnaire to identify symptoms consistent with functional gastrointestinal (GI) dysfunction. They also completed the Eating and Exercise Examination which collected data about behaviors including objective binge eating, self-induced vomiting, laxative use and excessive exercise.
Patients who were admitted to a specialty unit for those with eating disorders in Australia were studied and were found to have conditions such as anorexia nervosa, bulimia nervosa, polycystic ovarian syndrome, treated celiac disease, and treated bipolar depression. All of the 185 patients completed the Rome II Modular Questionnaire to identify symptoms consistent with functional gastrointestinal (GI) dysfunction. They also completed the Eating and Exercise Examination which collected data about behaviors including objective binge eating, self-induced vomiting, laxative use and excessive exercise.
Esophageal discomfort (heartburn and chest pain of non cardiac origin) was associated with excess exercise (more than 5 days/week). Self-induced vomiting was identified primarily in the patients diagnosed with bulimia. One interesting finding the researchers noted is that for patients who have disorder eating, pelvic floor symptoms that are not associated with functional constipation are a prominent feature. This data begs the question, how can we best screen for disordered eating in patients who present with bowel dysfunction that otherwise may fit with the symptoms and presentation of patients who do not have disordered eating?
Our first step may be to include important conditions and symptoms on our written or computer-based intake forms. Is “disordered eating” or bulimia, anorexia-nervosa included on your intake forms for patients? What about symptoms like heartburn, laxative use, or vomiting? (As an important aside, I always remember being surprised by a patient who had urinary incontinence when she told me that she leaked with vomiting. She had gone through a gastric bypass surgery and would vomit several times per week as a reaction to difficulty digesting food. There may be a few good reason therefore to include vomiting on a checklist.) As pelvic rehab providers, we can understand how frequent vomiting may lead to dehydration, intrabdominal and intrapelvic pressure, potential pelvic floor dysfunction, or how disordered eating may lead to other bowel dysfunctions such as constipation and/or fecal incontinence. If we also hold space for eating issues to be a concern, we may find that asking some valuable questions provides more information.
If you would like to learn more about nutrition and the pelvic health connections, you still have time to sign up for Megan Pribyl’s nutrition course which takes place in Lodi, California this June!.
Abraham, S., & Kellow, J. E. (2013) "Do the digestive tract symptoms in eating disorder patients represent functional gastrointestinal disorders?" BMC gastroenterology, 13(1), 1.