How does a male sports and orthopedic physical therapist come to teach about pelvic health and wellness? I was fortunate enough to spend ten years in the NHL as the physical therapist and athletic trainer for the Florida Panthers. Ice hockey is one of the sports that has the highest incidence of groin strains among other pelvic related pathologies.1 As a clinician that was responsible for taking care of the world’s best hockey players, I was challenged to understand the interconnected relationships between the lumbopelvic-hip complex very quickly.
 In the early years of my career development and the treatment of mostly males with pelvic pathologies, I leaned heavily on pelvic health professionals to help me understand an area of the body I received little training on in school and even less in my clinical care as a sports and orthopedic manual physical therapist. After years of treating hip and pelvic pathologies on my players I became more comfortable in this enigmatic area of the body. A good friend of mine was on faculty with Herman & Wallace and we frequently would communicate and compare notes. She was treating an increasing number of “sports hernias” (now termed athletic pubalgia or core muscle injury) and was relying on me to help her understand this injury and how to treat it. In turn, she helped me understand what went on in the pelvic health profession and what those therapists were trained to treat and how they went about it.
In the early years of my career development and the treatment of mostly males with pelvic pathologies, I leaned heavily on pelvic health professionals to help me understand an area of the body I received little training on in school and even less in my clinical care as a sports and orthopedic manual physical therapist. After years of treating hip and pelvic pathologies on my players I became more comfortable in this enigmatic area of the body. A good friend of mine was on faculty with Herman & Wallace and we frequently would communicate and compare notes. She was treating an increasing number of “sports hernias” (now termed athletic pubalgia or core muscle injury) and was relying on me to help her understand this injury and how to treat it. In turn, she helped me understand what went on in the pelvic health profession and what those therapists were trained to treat and how they went about it.
This collaboration eventually led to me joining Herman & Wallace and offering a sports and orthopedic perspective to pelvic floor consideration. I have attended Herman & Wallace’s Pelvic Floor courses to fully understand the training that a pelvic health therapist undergoes. Admittedly, I do not perform internal work because I have found a niche helping clinicians such as myself who understand that the pelvic floor is a key variable in human movement and we need to understand it at a much higher level than what we are exposed to in school, but don’t have the career trajectory of becoming an internal practitioner of the pelvic floor.
I have designed the Athletes and Pelvic Rehabilitation course to reach both the sports and orthopedic clinician as well as the pelvic health practitioner who might be a veteran of pelvic floor education and treatment. Both groups will leave this course with additional tools for their clinical tool box in the realms of manual therapy and exercise.2 Here are some of the objectives for the course:
- Provide clinicians that do not perform internal work a better and more complete understanding of how the pelvic floor integrates into human movement, particularly higher-level activities such as running, lifting and all types of sporting movements.
- Provide both the experienced and pelvic floor early learner with a comprehensive paradigm of exercise theory, development and progressions as well as how to provide non-internal manual therapy to influence the performance of the pelvic floor.
- Provide strategies for clinicians to determine when your patient would be better served with a referral to a pelvic health practitioner and what the current evidence is to support your decision.
- Create innovative and engaging therapeutic exercise programs (home exercise programs too!) for your patients directed at the pelvis with specific attention to upright and functional positioning.
What do people who have attended courses with Dr. Dischiavi have to say? Janna wrote the following email to Herman & Wallace about Steve's Course:
"Good morning. I wanted to make sure that you knew what a fantastic clinician you have to join your team in Steve Dischiavi. I am a practicing OB and orthopedic therapist and felt this course was fantastic! Usually the main goal is to come away with a couple of clinical "pearls." I felt as though I came away with a full days worth of "pearls." I really liked that the course was not totally pelvic floor based, however was totally relevant to the women's health population, but it will also apply to the majority of my current patient population as well. Thank you for the opportunity to learn from Steve!"
1. Orchard JW. Men at higher risk of groin injuries in elite team sports: a systematic review. Br J Sports Med. 2015;49(12):798-802.
2. Tuttle L. The Role of the Obturator Internus Muscle in Pelvic Floor Function. Journal of Women’s Health Physical Therapy. 2016;40(1):15-19.
In the spring of 2019, myself and two lab assistants will have the privilege of teaching PF1 to Kenyan physical therapists through the Kenya Medical Training College (KMTC) in Nairobi, Kenya. The program at KMTC started six years ago by Washington DC-based physical therapist Richard Jackson, and The Jackson Clinics Foundation (Teachandtreat.org), with a focus on orthopedic manual therapy. A neuro rehab program ensued two years later, and the aim for this women’s health program is to build a three level course series similar to the way it is taught in the United States. The goal of all of these programs is to transition them to Kenyan faculty within six years, which has recently occurred in the orthopedic component. Herman & Wallace Pelvic Rehab Institute has graciously agreed to donate curriculum content to the women’s health course component.
 Teaching assistant Terri Lannigan, PT, DPT, OCS, who has taught the lumbopelvic girdle course in the orthopedic program, and also practices women’s health physical therapy in the US, began laying the groundwork for this program with her students and in the Nairobi community last December. “Not only is there a tremendous need, but there is a lot of excitement from a group of students currently taking courses in the program, that women’s health education is coming to KMTC!”
Teaching assistant Terri Lannigan, PT, DPT, OCS, who has taught the lumbopelvic girdle course in the orthopedic program, and also practices women’s health physical therapy in the US, began laying the groundwork for this program with her students and in the Nairobi community last December. “Not only is there a tremendous need, but there is a lot of excitement from a group of students currently taking courses in the program, that women’s health education is coming to KMTC!”
Over the past month, I have been editing the Pelvic Floor 1 course to tailor it to our Kenyan physical therapist audience. The overwhelming majority of Kenyan PT’s do not have access to biofeedback or electric stim, so those sections will be omitted. As there are no documentation or coding requirements in the Kenyan health system, those sections of curriculum will also be edited out. Many of Terri’s PT students complained of significant underemployment, so we will keep the marketing component in our lectures, in hopes to promote expansion of women’s health PT to a larger segment of the Kenyan population.
Meanwhile, teaching assistant Kathy Golic, PT of Overlake Hospital Medical Center’s Pelvic Health Program in Bellevue, WA has headed up the data collection for a lecture on managing fistula and obstetric trauma. Kathy has accumulated data from many sources and conferred with several PTs currently involved in both clinical education as well as direct patient care in multiple African nations, to help us to create relevant, meaningful and culturally appropriate curriculum for this section of the PF1 course.
Pelvic Floor Level 1 will be offered between March 25 – April 6, 2019 at Kenya Medical Training College. We will post photos and additional information of our class and our experiences. We are grateful to Herman and Wallace and The Jackson Clinics Foundation for allowing us to be involved in this exciting endeavor.
Dustienne Miller MSPT, WCS, CYT is a Herman & Wallace faculty member, owner of Your Pace Yoga, and the author of the course Yoga for Pelvic Pain. Join her in Columbus, OH this April 27-28, to learn how yoga can be used to treat interstitial cystitis/painful bladder syndrome, vulvar pain, coccydynia, hip pain, and pudendal neuralgia. The course is also coming to Manchester, NH September 7-8, 2019, and Buffalo, NY on October 5-6, 2019.
How does a yoga program compare to a strength and stretching program for women with urinary incontinence? Dr. Allison Huang1 et al have published another research study, after publishing a pilot study2 on using group-based yoga programs to decrease urinary incontinence. Well-known yoga teachers Judith Hanson Lasater, PhD, and Leslie Howard created the yoga class and home program structure for this research study and the 2014 pilot study. The yoga program was primarily based on Iyengar yoga, which uses props to modify postures, a slower tempo to increase mindfulness, and pays special attention to alignment.
 To be chosen for this study, women had to be able to walk more than 2 blocks, transfer from supine to standing independently, be at least 50 years of age, and experience stress, urge, or mixed urinary incontinence at least once daily. Participants had to be new to yoga and holding off on clinical treatment for urinary incontinence, including pelvic health occupational and physical therapy.
To be chosen for this study, women had to be able to walk more than 2 blocks, transfer from supine to standing independently, be at least 50 years of age, and experience stress, urge, or mixed urinary incontinence at least once daily. Participants had to be new to yoga and holding off on clinical treatment for urinary incontinence, including pelvic health occupational and physical therapy.
28 women were assigned to the yoga intervention group and 28 women were assigned to the control group. The mean age was 65.4 with the age range of 55-83 years of age.
The control group received bi-weekly group class and home program instruction on stretching and strengthening without pelvic floor muscle cuing or relaxation training.
The yoga program met for group class twice a week for 90 minutes each and practiced at home one hour per week. The control group met twice a week for 90 minutes with a one-hour home program every week. Both groups met for 12 weeks.
Both groups received bladder behavioral retraining informational handouts. The information sheets contained education about urinary incontinence, pelvic floor muscle strengthening exercises, urge suppression strategies, and instructions on timed voiding.
The yoga program included 15 yoga postures: Parsvokonasana (side angle pose), Parsvottasana (intense side stretch pose), Tadasana (mountain pose) Trikonasana (triangle pose), Utkatasana (chair pose), Virabhadrasana 2 (warrior 2 pose), Baddha Konasana (bounded angle pose), Bharadvajasana (seated twist pose), Malasana (squat pose), Salamba Set Bandhasana (supported bridge pose), Supta Baddha Konasana (reclined cobbler’s pose), Supta Padagushthasana (reclined big toe pose), Savasana (corpse pose), Viparita Karani Variation (legs up the wall pose), and Salabhasana (locust pose).
Women in the yoga intervention group reported more than 76% average improvement in total incontinence frequency over the 3-month period. Women in the muscle stretching/strengthening (without pelvic floor muscle cuing and relaxation training) control group reported more than 56% reduction in leakage episodes.
Stress urinary incontinence episodes decreased by an average of 61% in the yoga group and 35% in the control group (P = .045). Episodes of urge incontinence decreased by an average of 30% in the yoga group and 17% in the control group (P = .77).
The take away? We know behavioral techniques have been shown to improve quality of life and decrease frequency and severity of urinary incontinence episodes.3 Couple this with our clinical interventions, and our patients have a way to reinforce the work we do in the clinic by themselves, or socially within their community. Yoga can be another tool in the toolbox for optimizing pelvic health.
1) Diokno AC et al. (2018). Effect of Group-Administered Behavioral Treatment on Urinary Incontinence in Older Women: A Randomized Clinical Trial. JAMA Intern Med.1;178(10):1333-1341. doi: 10.1001/jamainternmed.2018.3766.
2) Huang, Alison J. et al. (2019). A group-based yoga program for urinary incontinence in ambulatory women: feasibility, tolerability, and change in incontinence frequency over 3 months in a single-center randomized trial. American Journal of Obstetrics & Gynecology. 220(1) 87.e1 - 87.e13. doi: 10.1016/j.ajog.2018.10.031
3) Huang, A. J., Jenny, H. E., Chesney, M. A., Schembri, M., & Subak, L. L. (2014). A group-based yoga therapy intervention for urinary incontinence in women: a pilot randomized trial. Female pelvic medicine & reconstructive surgery, 20(3), 147-54.
In this post, we want to give a high-level overview of interstitial cystitis and an introduction to other resources if you’d like to dive deeper into treatment the condition. There’s a printable, patient-friendly version of this overview if you’d like to use it in describing the condition with patients. In addition, you may want to review the 8 Myths of Interstitial Cystitis series and the AUA Guidelines for Interstitial Cystitis.
Definition
Interstitial cystitis is defined as pain or pressure perceived to be related to the urinary bladder, associated with lower urinary tract symptoms of more than six weeks duration, in the absence of infection or other identifiable causes.
Unfortunately, for physicians, pelvic floor dysfunction falls under category of ‘unidentifiable cause.’ Interstitial cystitis is really more of a description of symptoms, rather than a discrete diagnosis, and the condition presents in many different ways.

Symptoms
The hallmarks of interstitial cystitis are pelvic pain, often in the suprapubic area or inner thighs, and urinary urgency and frequency. Other common symptoms include pain with intercourse, nocturia, low back pain, constipation, and urinary retention.
Many patients are surprised to realize that symptoms like painful intercourse, low back pain, and constipation are related to their IC diagnosis. This challenges the misconception that issues are arising solely from the bladder, and is a good way to help patients (and their physicians) understand that IC is about more than just the bladder.
Diagnosis
Interstitial cystitis is fundamentally a diagnosis of exclusion. Most patients suspect a urinary tract infection (UTI) when their symptoms first present. It’s actually common for symptoms to start as the result of a UTI, and simply not resolve once the infection has cleared. Patients are often treated with multiple rounds of antibiotics for these ‘phantom’ UTIs, where cultures have come back negative, before an IC diagnosis is considered.
It’s important for us as physical therapists to be able to share with patients that no testing is required to confirm an IC diagnosis, it can be diagnosed clinically. In practice, a urologist will likely want to conduct a cystoscopy, which can rule out more serious issues like bladder cancer as well as check for Hunner’s lesions (wounds in the bladder that are present in about 10% of IC patients). However, after that, no additional testing is needed. The potassium sensitivity test (PST) was formerly used by some urologists, but it has been shown to be useless diagnostically and extremely painful for patients and is not recommended by the American Urological Association. Urodynamic testing is also often conducted, but again is not necessary to establish an IC diagnosis.
Physical Therapy for IC
According to the American Urological Association, physical therapy is the most proven treatment for interstitial cystitis. It’s given an evidence grade of ‘A’ (the only treatment with that grade) and recommended in the first line of medical treatment.
In controlled clinical trials, manual physical therapy has been shown to benefit up to 85% of both men and women. These trials reported benefits after ten visits of one-hour treatment sessions.
In a study conducted at our clinic , PelvicSanity, we found that physical therapy was able to reduce pain for IC patients from an average of 7.6 (out of 10) before treatment to 2.6 following physical therapy. Similarly, how much their symptoms bothered patients fell from 8.3 to 2.8. More than half of patients reported improvements within the first three visits.
Unfortunately, many patients still aren’t referred to pelvic physical therapy by their physician. More than half of the patients in the study had seen more than 5 physicians before finding pelvic PT, and only 7% of patients felt they had been referred to physical therapy at the appropriate time by their doctor.
Multi-Disciplinary Approach
Patients with interstitial cystitis or pelvic pain always benefit from a multidisciplinary approach to treatment.This can include:
- Stress relief to downregulate the nervous system can decrease symptoms and reduce flares. Gentle exercise, meditation, yoga, deep breathing, or working with a psychologist can all provide benefits for patients.
- Diet and nutrition are important when working with IC patients. There is no formal ‘IC Diet’, but most patients are sensitive to at least a few trigger foods. The gold standard of treatment is an elimination diet, where the common culprits are completely removed from the diet and then added back in one at a time. This identifies which foods are triggers for patients. With nutrition for IC, patients should avoid their personal trigger foods and eat healthy – it doesn’t have to be any more restrictive or complicated.
- Alternative treatments like acupuncture have been shown to reduce pelvic pain in patients, and several supplements have shown benefits in trials or anecdotally among patients.
- Bladder treatments include instillations and nerve stimulation. Some patients may benefit from bladder instillations, but many others find that the process of the instillation actually causes additional symptoms. If instillations are beneficial, patients should be encouraged to address the underlying issues during the reprieve that instillations bring. Percutaneous tibial nerve stimulation or an implanted nerve stimulation device can both be possible treatment options.
- Oral medications can also reduce symptoms, but do not address the underlying cause of symptoms in patients. Medication that dampens the nervous system, often an anti-depressant or similar medication, can reduce pain and hypersensitivity. Anti-inflammatories may be beneficial in lowering inflammation and helping break the cycle of dysfunction-inflammation-pain. Most patients are started on Elmiron®, the only FDA-approved medication for IC; unfortunately, in the most recent clinical trial research Elmiron has been shown to be no more effective than a placebo. If it is effective, it only is beneficial for about one-third of patients, and many won’t be compliant with the drug due to cost and side effects.
 Nicole Cozean, PT, DPT, WCS (www.pelvicsanity.com/about-nicole) is the founder of PelvicSanity physical therapy in Southern California. Name the 2017 PT of the Year by the ICN, she’s the first physical therapist to serve on the Interstitial Cystitis Association’s Board of Directors and the author of the award-winning book The IC Solution (www.pelvicsanity.com/the-ic-solution). She teaches at her alma mater, Chapman University, as well as continuing education through Herman & Wallace. Nicole also founded the Pelvic PT Huddle (www.facebook.com/groups/pelvicpthuddle), an online Facebook group for pelvic PTs to collaborate.
Nicole Cozean, PT, DPT, WCS (www.pelvicsanity.com/about-nicole) is the founder of PelvicSanity physical therapy in Southern California. Name the 2017 PT of the Year by the ICN, she’s the first physical therapist to serve on the Interstitial Cystitis Association’s Board of Directors and the author of the award-winning book The IC Solution (www.pelvicsanity.com/the-ic-solution). She teaches at her alma mater, Chapman University, as well as continuing education through Herman & Wallace. Nicole also founded the Pelvic PT Huddle (www.facebook.com/groups/pelvicpthuddle), an online Facebook group for pelvic PTs to collaborate.
Interstitial Cystitis Course
In our upcoming course for physical therapists in treating interstitial cystitis (April 6-7, 2019 in Princeton, New Jersey), we’ll focus on the most important physical therapy techniques for IC, home stretching and self-care programs, and information to guide patients in creating a holistic treatment plan. The course will delve into how to handle complex IC presentations. It’s a deep dive into the condition, focusing not just on manual treatment techniques but also how to successfully manage an IC patient from beginning to resolution of symptoms.
Additional Resources
- Interstitial Cystitis Overview (printable)
- The Interstitial Cystitis Solution
- Patient groups include the Interstitial Cystitis Association (ICA) (www.ic-help.org) and the IC Network (www.ic-network.com), which both have fantastic resources for patients.
- The AUA Guidelines for IC
- IC Flare-Busting Plan
The need for artful incorporation of Hippocrates’ wisdom is great in today’s healthcare landscape. As conversation of nutrition broadens into multidisciplinary fields, his wisdom resonates: first, “we must make a habit of two things; to help; or at least to do no harm”. Second, we must modernize the ancient adage: “let food be thy medicine and let medicine be thy food”. And finally, health care providers will do well to be guided by his insight that “all disease starts in the gut”. Hippocrates’ keen observations during his era, modern science is confirming, hold keys to the plight of our times as we seek to find better ways to manage complex conditions commonly encountered in pelvic rehab practice settings and beyond.
 Considered some of the oldest writings on medicine, the “Hippocratic Corpus” is a collection of more than 60 medical books attributed directly and indirectly to Hippocrates himself who lived from approximately 460 to 377 BCE.2 According to the Corpus, Hippocratic approach recommends physical exercise and a “healthy diet” as a remedy for most ailments - with plants being prized for their healing properties. If -during illness states - employment of nourishment and movement strategies fail, then medicinal considerations could be made. This logos - the ancient Greek word for logic - is the art of reason whose relevance today is perhaps more poignant than in ancient times.
Considered some of the oldest writings on medicine, the “Hippocratic Corpus” is a collection of more than 60 medical books attributed directly and indirectly to Hippocrates himself who lived from approximately 460 to 377 BCE.2 According to the Corpus, Hippocratic approach recommends physical exercise and a “healthy diet” as a remedy for most ailments - with plants being prized for their healing properties. If -during illness states - employment of nourishment and movement strategies fail, then medicinal considerations could be made. This logos - the ancient Greek word for logic - is the art of reason whose relevance today is perhaps more poignant than in ancient times.
In this logos, by making a habit of helping, or at the very least, not harming, it becomes particularly important to identify the unique nutritional landscape that surrounds us. The Hippocratic Oath emanates reason. It is logical that we would seek to practice (healthcare) to the best of our ability, share knowledge with other providers, employ sympathy, compassion and understanding, and help in disease prevention whenever possible.2 One of the most helpful and powerful aspects of rehabilitation is the gift of time we have for meaningful and instructional conversation with our clients. Our interactions with clients can and should address the realm of nutrition as it relates to the health of the mind and body. Because, after all - to help - is why many become health care providers in the first place.
Detailing a “healthy diet” in Hippocratic times was certainly simpler, as the uncontrolled variable of processed foods- as we know them- did not exist. Therefore, we reflect upon the quote: “let food be thy medicine and let medicine be thy food” and acknowledge that this modern food landscape is vastly different 1 than in ancient times. Compounding the issue, our standard logic for helping has gotten somewhat out of order. And both medicine and food carry meanings today reflective of modern times. The issues of poly-pharmacy and the tragedy of medically prescribed unintentional overdoses (or intolerances) remind us of our ‘medicine first’ mentality and the unfortunate reality that medicine is not the cure-all we so wish it could be. Further, not all ‘food’ today is food. Real food sustains and nourishes us. Real food can also heal. We need to celebrate real food for being real food, and champion it’s miraculous ability to support, heal, and transform the human condition.
Finally, health care providers will do well to be guided by Hippocratic insight that “all disease starts in the gut” and to logically extrapolate the opposite: much healing can begin in the gut. It is through this ancient concept that we can organize our modern science and begin to concretely and intentionally help heal ourselves and others from the inside out. Once we understand the key role of digestion and our gut on our health and well-being, the rest is pure logic. We simply need a map for navigation of these universal concepts to go along with our renewed appreciation for the art of reason.
Let Nutrition Perspectives for the Pelvic Rehab Therapist help provide this map. Evolve your nutritional logos into a beautiful and nourishing framework by joining the hundreds of pelvic rehab therapists and other health care providers who have attended Nutrition Perspectives in Pelvic Rehab. Be inspired and empowered on your integrative journey. Live courses will be offered at three sites in 2019: March 1-3 in Arlington, VA, June 7-9 in Houston, TX, and October 11-13 in Tampa, FL!
Fardet, A., Rock, E., Bassama, J., Bohuon, P., Prabhasankar, P., Monteiro, C., . . . Achir, N. (2015). Current food classifications in epidemiological studies do not enable solid nutritional recommendations for preventing diet-related chronic diseases: the impact of food processing. Adv Nutr, 6(6), 629-638. doi:10.3945/an.115.008789
Biography.com https://www.biography.com/people/hippocrates-082216. Accessed January 11, 2019.
When It Comes to Bone Building Activities for Osteoporosis, there’s Weight Bearing and then there’s Weight Bearing!
Ask just about anyone on the street what one should do for osteoporosis and the typical answer is- weight bearing exercises. And they would be partially right. Weight bearing, or loading activities have been shown to increase bone density.1 But that’s not the whole story.
 Regarding weight bearing exercises, the million-dollar question is, “How much weight bearing is enough to stimulate bone growth and how much is too much to compromise bone at risk for a fracture? We know that there are incidents of individuals fracturing from just their own body weight upon standing. Recently patients have been asking about heel drops and stomping, and whether they should do them. One size does not fit all.
Regarding weight bearing exercises, the million-dollar question is, “How much weight bearing is enough to stimulate bone growth and how much is too much to compromise bone at risk for a fracture? We know that there are incidents of individuals fracturing from just their own body weight upon standing. Recently patients have been asking about heel drops and stomping, and whether they should do them. One size does not fit all.
An alternative is to focus on “odd impact” loading. A study by Nikander et al 2 targeted female athletes in a variety of sports classified by the type of loading they apparently produce at the hip region; that is, high-impact loading (volleyball, hurdling), odd-impact loading (squash-playing, soccer, speed-skating, step aerobics), high magnitude loading (weightlifting), low-impact loading (orienteering, cross-country skiing), and non-impact loading (swimming, cycling). The results showed high-impact and odd-impact loading sports were associated with the highest bone mineral density.
Morques et al, in Exercise Effects on Bone Mineral Density in Older Adults: A meta-analysis of randomized controlled trials, found that odd impact has potential for preserving bone mass density as does high impact in older women. Activities such as side stepping, figure eights, backward walking, and walking in square patterns help “surprise the bones” due to different angles of muscular pull on the hip. The benefit, according to Nikander, is that we can get the same osteogenic benefits with less force; moderate versus high impact. This type of bone training would offer a feasible basis for targeted exercise-based prevention of hip fragility. I tell my osteoporosis patients that if they walk or run the same route, the same distance, and the same speed that they are not maximizing the osteogenic benefits of weight bearing. Providing variety to the bones creates increased bone mass in the femoral neck and lumbar spine.4
Dancing is another great activity which combines forward, side, backward, and diagonal motions to movement. In addition, it adds music to make the “weight bearing exercises” more fun. Due to balance and fall risk many senior exercise classes offer Chair exercise to music. Unfortunately sitting is the most compressive position for the spine and is particularly problematic with osteoporosis patients. Also the hips do not get any weight bearing benefit. Whenever safely possible, have patients stand; you can position two kitchen chairs on either side, much like parallel bars, to hold on to while they “dance.”
Providing creativity in weight bearing activities using odd impact allows not only for fun and stimulation; it also offers more “bang for the buck!”
- Mosekilde L. Age-related changes in bone mass, structure, and strength--effects of loading. Z Rheumatol (2000); 59 Suppl 1:1-9.
- Nikander et al. Targeted exercises against hip fragility. Osteoporosis International (2009)
- Marques et al. Exercise effects on bone mineral density in older adults: a meta-analysis of randomized controlled trials. Epub 2011 Sep 16
- Weidauer L. et al. Odd-impact loading results in increased cortical area and moments of inertia in collegiate athletes. Eur J Appl Physiol (2014)
Andrea Wood, PT, DPT, WCS, PRPC is a pelvic health specialist at the University of Miami downtown location. She is a board certified women’s health clinical specialist (WCS) and a certified pelvic rehabilitation practitioner (PRPC). She is passionate about orthopedics and pelvic health. In her spare time, you can find her enjoying the south Florida outdoors.
Inflammatory bowel disease (IBD) includes the two diagnosis of Crohn’s Disease and Ulcerative Colitis. While both can cause similar health effects, the differences of the disease pathologies are listed below:1
| Ulcerative Colitis | Crohn’s Disease | |
| Affected Area |
|
|
| Pattern of Damage |
|
|
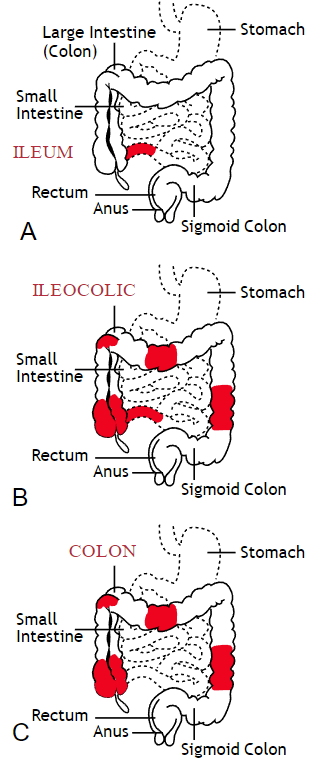
Common complications experienced by patients with IBD include fecal incontinence, fecal urgency, night time soiling, urinary incontinence, abdominal pain, hip and core weakness, pelvic pain, fatigue, osteoporosis, and sarcopenia. In a sample of 1,092 patients with Crohn’s Disease, Ulcerative Colitis, or unclassified IBD, 57% reported fecal incontinence. Fecal incontinence was reported not only during periods of flare ups, but also during remission periods.2 One common factor affecting fecal incontinence is external anal sphincter fatigue. External anal sphincter fatigue has also been shown to be present in IBD patients who are not experiencing fecal incontinence or fecal urgency. IBD patients have been shown in studies to have similar baseline pressures versus healthy matched controls, thus indicating the possibility that deficits in endurance versus strength can play a larger role in fecal incontinence.3 Other factors contributing to fecal incontinence include post inflammatory changes that may alter anorectal sensitivity, anorectal compliance, neuromuscular coordination, and cause visceral hypersensitivity. Visceral hypersensitivity may be caused by continuous release of inflammatory mediators found in patients with IBD. It is also important to screen properly for incomplete bowel emptying and stool consistency to rule out overflow diarrhea or fecal impaction. Reports of need to splint digitally for full evacuation may indicate incomplete bowel emptying and defaectory disorders such as paradoxical contraction of the puborectalis muscle or rectocele. Anorectal manometry testing may be highly useful in identifying patients likely to improve from biofeedback therapy.4
Urinary incontinence can also be another secondary consequence to IBD. In a sample of 4,827 patients with IBD, 1/3 of responders reported urinary incontinence that was strongly associated with the presence of fecal incontinence. Frequent toilet visits for defecation may stimulate overactive bladder. Women were more likely to experience fecal incontinence versus men. One possible mechanism for increased fecal incontinence in women is men often have a longer and more complete anal sphincter that may be protective of fecal incontinence.5
Physical activity has been shown to be lower in patients with IBD versus healthy controls. 6, 7 Guiding IBD patients in proper exercises programs can have great benefits. Exercise may reduce inflammation in the gut and maintain the integrity of the intestines, reducing inflammatory bowel disease risk.8 It can also help increase bone mass density, an important factor in IBD patients who are at greater risk for osteoporosis. It has also been shown to help general fatigue in IBD patients. Patients with Crohn’s disease who participate in higher exercise levels may be less likely to develop active disease at 6 months. Treadmill training at 60% VO2 max and running three times a week has not been shown to evoke gastrointestinal symptoms in IBD patients. An increase of BMI predicts poorer outcomes and shorter time to first surgery in patients with Crohn’s disease.6
Conservative physical therapy interventions for treating IBD symptoms can include the following:
| Symptoms resulting from IBD | Physical Therapy Interventions |
| Fecal Incontinence (FI) |
|
| Urinary urgency |
|
| Sarcopenia |
|
| Fatigue |
|
| Pelvic Pain |
|
Surgical interventions for IBD are dependent upon what type of disease the patient has and what areas of the intestines are affected the most. Surgery may be considered once the disease has become non responsive to medication therapy and quality of life continues to decline. A colectomy involves removing the colon while a proctocolectomy involves both removal of the colon and rectum. For ulcerative colitis patients, options include total proctocolectomy with end ileostomy or a restorative proctocolectomy with ileal pouch anal anastomosis. Restorative proctocolectomy eliminates the need for an ostomy bag making it the preferred surgery of choice if possible and gold standard for ulcerative colitis patients.10 For patients with Crohn’s disease, options include resection of part of the intestines followed by an anastomosis of the remaining healthy ends of the intestines, widening of the narrowed intestine in a procedure called a strictureplasty, colectomy or proctocolectomy, fistula repair, and removal of abscesses if needed.11
1. Crohn’s and Colitis Foundation. 2019. What is Crohn’s Disease. Retrieved from: http://www.crohnscolitisfoundation.org/what-are-crohns-and-colitis/what-is-crohns-disease/
2. Vollebregt PF, van Bodegraven A, Markus-de Kwaadsteniet T, et al. Impacts on perianal disease and faecal incontinence on quality of life and employment in 1092 patients with inflammatory bowel disease. Ailment Pharmacol Ther. 2018; 47: 1253-1260
3. Athanasios A, Kostantinos H, Tatsioni A et al. Increased fatigability of external anal sphincter in inflammatory bowel disease: significance in fecal urgency and incontinence. J Crohns Colitis (2010) 4: 553-560.
4. Nigam G, Limdi J, Vasant D. Current perspectives on the diagnosis and management of functional anorectal disorders in patients with inflammatory bowel disease. Therap Adv Gastroenterol. 2018 Dec 6: doi: 10.1177/1756284818816956
5. Norton C, Dibley L, Basset P. Faecal incontinence in inflammatory bowel disease: Associations and effect on quality of life. J Crohn’s Colitis. (2013) 7, e302-e311.
6. Biliski J, Mazur-Bialy A, Brzozowski B et al. Can exercise affect the course of inflammatory bowel disease? Experimental and clinical evidence. Pharmacological Reports. 2016 (68): 827-836.
7. Tew G, Jones K, Mikocka-Walus A. Physical activity habits, limitations, and preditors in people with inflammatory bowel disease: a large cross-sectional online survey. Inflamm Bowel Dis. 2016; 22(12): 2933-2942.
8. Vincenzo M, Villano I, Messina A. Exercise modifies the gut microbiota with positive health effects. Oxidative Medicine and Cellular Longevitiy. 2017: Article ID 3831972.
9. Cramer H, Schafer M, Schols M. Randomised clinical trial: yoga vs written self care advice for ulcerative colitis. Aliment Pharmacol Ther. 2017; 45: 1379-1389.
10. Cornish J, Wooding K, Tan E, et al. Study of sexual, urinary, and fecal function in females following restorative proctocolectomy. Inflamm Bowel Dis. 18 (9) 2012. 1601-160
11. Crohn’s and Colitis Foundation. 2019. Surgery Options. Retrieved from: http://www.crohnscolitisfoundation.org/what-are-crohns-and-colitis/what-is-crohns-disease/surgery-options.html
Most people are told that inguinal hernia repair is a low risk surgery. While death or severe injury is rare, penile or testes pain after hernia repair is not a novel or recent finding. In 1943, Magee first discussed patients having genitofemoral neuralgia after appendix surgery. By 1945, both Magee and Lyons stated that surgical neurolysis gave relief of genital pain following surgical injury (neurolysis is a surgical cutting of the nerve to stop all function). However, it should be noted that with neurolysis, sensory loss will also occur, which is an undesired symptom for sexual function and pleasure. In 1978 Sunderland stated genitofemoral neuralgia was a well-documented chronic condition after inguinal hernia repair.
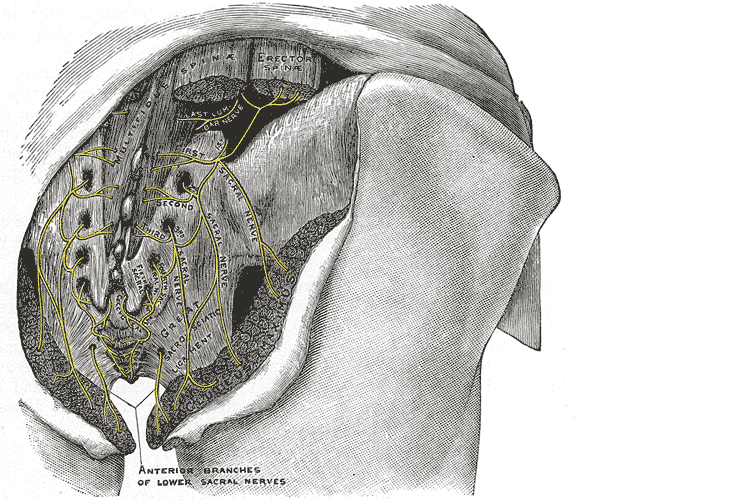 Let’s do a quick anatomy review. The inguinal canal is located at the lower abdomen and is actually an extension of the external oblique muscles. Is travels along the line from the ASIS to the pubic tubercle, occupying grossly the medial third of this segment. It has a lateral ring where contents from the abdomen exit and a medial ring where the contents of the canal exit superficially. This ring contains the spermatic cord (male), round ligament (female), as well as the ilioinguinal and genitofemoral nerves. For males, in early life, the testes descend from the abdominal cavity to the exterior scrotal sac through the inguinal canal, bringing a layer of the obliques, transverse abdominus, and transversalis fascia with them within the first year of life. Just as a female can experience prolapse from prolonged increased intra-abdominal pressure, a male can have a herniation through the anterior abdominal wall and inguinal canal with increased abdominal pressure. Such pressure inducing activities can be lifting, coughing, and sports activities. When this occurs, an inguinal hernia repair is generally indicated. Because the genitofemoral nerve is within the contents of the inguinal canal, it can be susceptible to surgery in this area. The genitofemoral nerve has sensory innervation to the penis and testes and is responsible for the cremasteric reflex. Symptoms of genitofemoral neuralgia in men can be penis or testes pain, numbness, hypersensitivity, and decreased sexual satisfaction or function.
Let’s do a quick anatomy review. The inguinal canal is located at the lower abdomen and is actually an extension of the external oblique muscles. Is travels along the line from the ASIS to the pubic tubercle, occupying grossly the medial third of this segment. It has a lateral ring where contents from the abdomen exit and a medial ring where the contents of the canal exit superficially. This ring contains the spermatic cord (male), round ligament (female), as well as the ilioinguinal and genitofemoral nerves. For males, in early life, the testes descend from the abdominal cavity to the exterior scrotal sac through the inguinal canal, bringing a layer of the obliques, transverse abdominus, and transversalis fascia with them within the first year of life. Just as a female can experience prolapse from prolonged increased intra-abdominal pressure, a male can have a herniation through the anterior abdominal wall and inguinal canal with increased abdominal pressure. Such pressure inducing activities can be lifting, coughing, and sports activities. When this occurs, an inguinal hernia repair is generally indicated. Because the genitofemoral nerve is within the contents of the inguinal canal, it can be susceptible to surgery in this area. The genitofemoral nerve has sensory innervation to the penis and testes and is responsible for the cremasteric reflex. Symptoms of genitofemoral neuralgia in men can be penis or testes pain, numbness, hypersensitivity, and decreased sexual satisfaction or function.
In 1999 Stark et al noted pain reports as high as 63% post hernia repair. The highest rates of genitofemoral neuralgia are reported with laparoscopic or open hernia repair (Pencina, 2001). The mechanism for GF neural entrapment is entrapment within scar or fibrous adhesions and parasthesia along the genitofemoral nerve (Harms 1984, Starling and Harms 1989, Murovic 2005, and Ducic 2008). It is well known that scar and adhesion densify and visceral adhesions increase for years after surgery. Thus, symptoms can increase long after the surgery or may take years to develop. In 2006, Brara postulated that mesh in the region can contribute to subsequent genitofemoral nerve tethering which can be exacerbated by mesh in the inguinal or the retroperitoneal space. With an anterior mesh placement, there is no fascial protection left for the genitofemoral nerve.
Genitofemoral neuralgia is predominately reported as a result of iatrogenic nerve damage during surgery or trauma to the inguinal and femoral regions (Murovic et al, 2005). However, genitofemoral neuropathy can be difficulty and elusive to diagnose due to overlap with other inguinal nerves (Harms, 1984 and Chen 2011).
In my clinical experience, I have seen such symptoms after hernia repair, but also after procedures near the inguinal region such as femoral catheters for heart procedures, appendectomies, and occasionally after vasectomy.
As a pelvic PT, what are we to do with this information? First off, we can realize that all pelvic neuropathy is not necessarily due to the pudendal nerve. In the anterior pelvis, there is dual innervation from the inguinal nerves off the lumbar plexus as well as the dorsal branch of the pudendal nerve. When patients have a history of inguinal hernia repair, we can consider the genitofemoral nerve as a source of pain. Medicinally, the only research validated options for treatment are meds such as Lyrica or Gabapentin that come with drowsiness, dizziness and a score of side effects. Surgically neurectomy or neural ablation are options with numbness resulting, however, many patients do not want repeated surgery or numbness of the genitals. As pelvic therapists, we can manually fascially clear the path of the nerve from L1/L2, through the psoas, into and out of the canal and into the genitals. We can also manually directly mobilize the nerve at key points of contact as well as doing pain free sliders and gliders and then give the patient a home program to maintain mobility. Pelvic manual therapy can offer a low risk, side-effect free option to ameliorate the sequella of inguinal hernia repair. Come join us at Lumbar Nerve Manual Assessment and Treatment in Chicago this Spring to learn how to effectively treat all the nerves of the lumbar plexus.
Cesmebasi, A., Yadav, A., Gielecki, J., Tubbs, R. S., & Loukas, M. (2015). Genitofemoral neuralgia: a review. Clinical Anatomy, 28(1), 128-135.
Lyon, E. K. (1945). Genitofemoral causalgia. Canadian Medical Association Journal, 53(3), 213.
Magee, R. K. (1943). Genitofemoral Causalgia: New Syndrome. The Journal of Nervous and Mental Disease, 98(3), 311.
Sunderland S. Nerves and nerve injuries. 2nd ed. Edinburgh: Churchill Livingstone, 1978
Today's guest post comes from Kelsea Cannon, PT, DPT, a pelvic health practitioner in Seattle, WA. Kelsea graduated from Des Moines University in 2010 and practices at Elizabeth Rogers Pilates and Physical Therapy.
Many studies done on pelvic floor muscle training largely have subjects who are Caucasian, moderately well educated, and receive one-on-one individualized care with consistent interventions. This led a group of researchers to investigate the occurrence of pelvic floor dysfunction, specifically pelvic organ prolapse (POP), in parous Nepali women. These women are known to have high incidences of POP and associated symptomology. Another impetus to perform this research: the discovery that there was a major lack of proper pelvic floor education for postpartum women. These women were commonly encouraged to engage their pelvic floor muscles via performing supine double leg lifts, sucking in their tummies/holding their breath/counting to ten, and squeezing their glutes. These exercises would be on a list of no-no’s here in the United States. In 2017, Delena Caagbay and her team of researchers discovered that in Nepal, no one really knew the correct way to teach proper pelvic floor muscle contractions, preventing the opportunity for women to better understand their pelvic floors. The team then set out to investigate the needs of this population, with the eventual goal of providing effective pelvic floor education for Nepali women.
 Caagbay and her team first wanted to know what baseline muscle activity the Nepali women had in their pelvic girdle. Physical examinations and internal pelvic floor muscle strength assessments revealed that surprisingly there was a low prevalence of pelvic floor muscle defects, such as levator avulsions and anal sphincter trauma. Uterine prolapses were most common while rectoceles were comparatively less common. Their muscles were also strong and well-functioning, often averaging a 3/5 on the Modified Oxford Scale. It was hypothesized that these women had low prevalence of muscle injury because instruments were not commonly used during childbirth, they had lower birth weight babies, and the women were typically younger when giving birth (closer to 20-21 years old). But they had a high prevalence of POP even with good muscle tone? Researchers suggested that their incidence of POP is likely stemming from their sociocultural lifestyle requirements, as women are left to do most of the daily household chores and caregiving tasks while men often travelled away from the home to perform paid labor. Physical responsibilities for these women commonly begin at younger ages and while it helps promote good muscle tone, the heavier loading places pressure on the connective tissue and fascia that support the pelvic organs. Because of the demands of their lifestyles, Nepali women are often forced to return to their physically active state within a couple weeks after giving birth.
Caagbay and her team first wanted to know what baseline muscle activity the Nepali women had in their pelvic girdle. Physical examinations and internal pelvic floor muscle strength assessments revealed that surprisingly there was a low prevalence of pelvic floor muscle defects, such as levator avulsions and anal sphincter trauma. Uterine prolapses were most common while rectoceles were comparatively less common. Their muscles were also strong and well-functioning, often averaging a 3/5 on the Modified Oxford Scale. It was hypothesized that these women had low prevalence of muscle injury because instruments were not commonly used during childbirth, they had lower birth weight babies, and the women were typically younger when giving birth (closer to 20-21 years old). But they had a high prevalence of POP even with good muscle tone? Researchers suggested that their incidence of POP is likely stemming from their sociocultural lifestyle requirements, as women are left to do most of the daily household chores and caregiving tasks while men often travelled away from the home to perform paid labor. Physical responsibilities for these women commonly begin at younger ages and while it helps promote good muscle tone, the heavier loading places pressure on the connective tissue and fascia that support the pelvic organs. Because of the demands of their lifestyles, Nepali women are often forced to return to their physically active state within a couple weeks after giving birth.
After assessing the current needs, cultural norms, and prevalence of POP in Nepali women, Caagbay et al created an illustrative pamphlet on how to contract pelvic floor muscles as well as provided verbal instruction on pelvic floor muscle activation. Transabdominal real time ultrasound was applied to assess the muscle contraction of 15 women after they received this education. Unfortunately, even after being taught how to engage their pelvic floor muscles, only 4 of 15 correctly contracted their pelvic floors.
This study highlighted that brief verbal instruction plus an illustrative pamphlet was not sufficient in teaching Nepali women how to correctly contract their pelvic floor muscles. Although there was a small sample size, these results can likely be extrapolated to the larger population. Further research is needed to determine how to effectively teach correct pelvic floor muscle awareness to women with low literacy and/or who reside in resource limited areas. Lastly, it is important to consider the significance of fascial and connective tissue integrity within the pelvic floor when addressing pelvic organ prolapse.
1 Can a leaflet with brief verbal instruction teach Napali women how to correctly contract their pelvic floor muscles? DM Caagbay, K Black, G Dangal, C Rayes-Greenow. Journal of Nepal Health Research Council 15 (2), 105-109.
https://www.nepjol.info/index.php/JNHRC/article/viewFile/18160/14771
2 Pelvic Health Podcast. Lori Forner. Pelvic organ prolapse in Nepali women with Delena Caagbay. May 31, 2018.
3 The prevalence of pelvic organ prolapse in a Nepali gynecology clinic. (2017) F. Turel, D. Caagbay, H.P. Dietz. Department of Obstetrics and Gynecology, Sydney Medical School Napean, University of Sydney.
4 The prevalence of major birth trauma in Nepali women. (2017) F. Turel, D. Caagbay, H.P. Dietz. Department of Obstetrics and Gynecology, Sydney Medical School Nepean, University of Sydney.
Going to the Combined Sections Meeting of the American Physical Therapy Association (CSM2019)? Look for Herman & Wallace instructor Carolyn McManus, MPT, MA at the educational session titled “Pain Talks: Conversations with Pain Science Leaders on the Future of the Field”. Carolyn will be a panelist along with Kathleen Sluka, PT, PhD, Steve George, PT, PhD, Carol Courtney, PT, PhD and Adriaan Louw, PT, PhD. The panel will be moderated by Derrick Sueki, DPT, PhD and Mark Shepherd, DPT, OCS.
 These influential leaders will share how they personally became interested in the field of pain and discuss innovative pain treatment, as well as leading edge pain research and its translation into clinical practice. Initiatives to standardize entry-level curriculum, develop pathways to pain specialization and create post-professional opportunities such as pain-specific residencies and fellowships will be explored. The session will conclude with the leaders discussing their views on the future of pain and the role of physical therapy in its management. The audience will be able to submit questions via text or email to the moderator for individual or panel discussion.
These influential leaders will share how they personally became interested in the field of pain and discuss innovative pain treatment, as well as leading edge pain research and its translation into clinical practice. Initiatives to standardize entry-level curriculum, develop pathways to pain specialization and create post-professional opportunities such as pain-specific residencies and fellowships will be explored. The session will conclude with the leaders discussing their views on the future of pain and the role of physical therapy in its management. The audience will be able to submit questions via text or email to the moderator for individual or panel discussion.
We are thrilled to have Carolyn on our faculty and excited that she has been offered this honor to contribute insights from her over 30-year career experience in the field of pain with her colleagues at CSM2019. Carolyn will offer her popular courses, Mindfulness for Rehabilitation Professionals at University Hospitals in Cleveland, OH on April 6 and 7, and Mindfulness-Based Pain Treatment in Portland, OR May 18 and 19, and Houston TX, October 26 and 27. We recommend these unique opportunities to train with a nationally recognized leader who pioneered the successful applications of mindfulness to the field of physical therapy. Hope to see you there!
By accepting you will be accessing a service provided by a third-party external to https://hermanwallace.com/








































